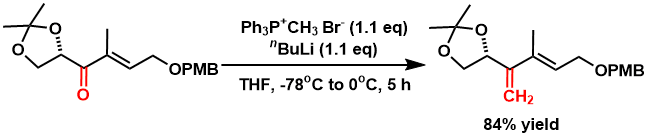- Generality
- Reagent Availabiltiy
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
Compounds in which the adjacent anion is stabilized by a heteroatom with a positive formal charge are generally called ylides. The synthesis of alkenes from carbonyl compounds using phosphorous ylides is called the Wittig reaction.
Olefin synthesis based on classical E2 elimination conditions generally requires extreme conditions such as strong acid/base and/or high reaction temperature, where the formation of regioisomer and double bond isomerization are frequently problematic. In contrast, the Wittig reaction converts carbonyl groups to C=C bonds at low temperatures. Also, the stereoisomerism is well controllable. Because of these benefits, it is still widely used as a first choice for synthesizing alkenes from carbonyls.
A major drawback of the Wittig reaction is that removing the phosphine oxide byproduct is sometimes difficult. In the improved methods, such as the Wittig-Horner method (using phosphite instead of triphenylphosphine) and the Horner-Wadsworth-Emmons reaction, the phosphorous byproducts are highly polar and water-soluble and thus easy to remove.
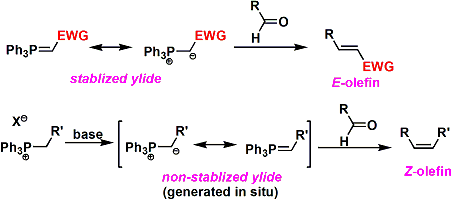
Electron-deficient phosphorus ylides are stable ylides and can be isolated. The reaction with stable ylides yields E-olefins. On the other hand, phosphorus ylides that cannot be isolated (decompose in water or air) are called unstable ylides. These are prepared by treating phosphonium salts with a base. In the reaction with unstable ylides, Z-olefins are formed.
-
General References
- Wittig, G.; Scholkopf, U. Ber. 1954, 87, 1318. doi:10.1002/cber.19540870919
- Wittig, G.; Scholkopf, U. Ber. 1955, 88, 1654. doi:10.1002/cber.19550881110
<Schlosser Modification>
- Schlosser, M.; Christmann, K. F. Angew. Chem. Int. Ed. Engl. 1966, 5, 126. doi:10.1002/anie.196601261
<Mechanism>
- Byrnea, P. A.; Gilheany, D. G. Chem. Soc. Rev. 2013, 42, 6670. doi:10.1039/C3CS60105F
<review>
- Maercker, A. Org. React. 1965, 14, 270.
- Vollhardt, K. P. C. Synthesis 1975, 765. DOI: 10.1055/s-1975-23920
- Becker, K. B. Tetrahedron 1980, 36, 1717. doi:10.1016/0040-4020(80)80068-8
- Murphy, P. J.; Brennan, J. Chem. Soc. Rev. 1988, 17, 1. DOI: 10.1039/CS9881700001
- Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863. DOI: 10.1021/cr00094a007
- Cristau, H.-J. Chem. Rev. 1994, 94, 1299. DOI: 10.1021/cr00029a006
- Burton, D. J.; Yang, Z.-Y.; Qiu, W. Chem. Rev. 1996, 96, 1641. DOI: 10.1021/cr941140s
- Nicolaou, K. C.; Harter, M. W.; Gunzner, J. L.; Nadin, A. Liebigs Ann. 1997, 7, 1283. DOI: 10.1002/jlac.199719970704
- Murphy, P. J.; Lee, S. E. J. Chem. Soc. Perkin Trans. 1 1999, 3049. DOI: 10.1039/A803560A
- Hoffmann, R. W. Angew. Chem. Int. Ed. 2001, 40, 1411. [abstract]
- Rein, T.; Pedersen, T. M. Synthesis 2002, 579. DOI: 10.1055/s-2002-23535
- Xu, S.; He, Z. RSC Adv. 2013, 3, 16885. DOI: 10.1039/C3RA42088D
-
History
Georg Wittig at the University of Heidelberg developed this reaction. Together with H.C. Brown, he was awarded the Nobel Prize in Chemistry in 1979 for their contributions to synthetic organic chemistry using organophosphorus.

Georg Wittig (nobelprize.org)
-
Reaction Mechanism
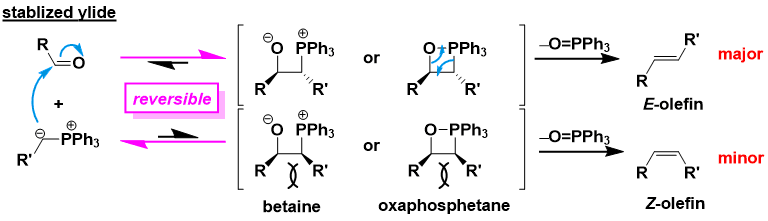
First, a phosphine ylide attacks a carbonyl carbon to give a betaine intermediate or an oxaphosphetane intermediate. The phosphine oxide is subsequently removed to give an alkene. The strong phosphorus-oxygen bond is the driving force for the reaction to proceed. For the oxaphosphetane intermediate, a stabilized version has been isolated and structurally determined by a suitable ligand design, while a betaine intermediate has not been identified to date, and its existence is speculative.
In the case of stable ylides, the carbonyl + phosphorus ylide addition step is reversible. The reaction proceeds through the oxaphosphetane intermediate, which is the most stable under thermodynamic control. Thus, E-olefin is the main product.
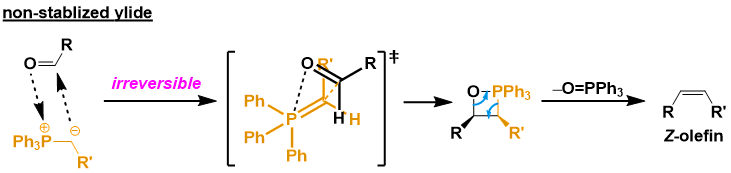
In the case of unstable ylides, the reagent is highly reactive, and the addition of the carbonyl + phosphorus ylide proceeds irreversibly. Therefore, the oxaphosphetane intermediate is formed via a kinetically favored transition state. Specifically, the four-membered ring transition state (below figure) is the least sterically repulsive. Subsequent phosphine oxide elimination gives the Z-olefin. Note that the presence of lithium salts in the system reduces Z-selectivity.
-
Examples
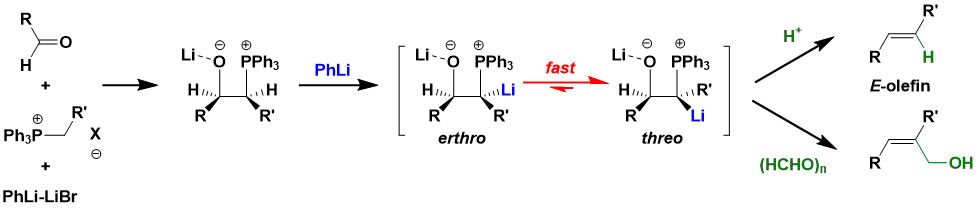
When the intermediate produced by the low-temperature addition of phosphorus ylides to aldehydes is treated with a strong base such as PhLi, a β-oxide ylide is formed. It is known that this quickly transitions to the thermodynamically stable threo form. This can be utilized to synthesize E-olefins from unstable ylides (Schlosser method). β-Oxido ylides also react with a variety of electrophiles and can be multifunctionalized.
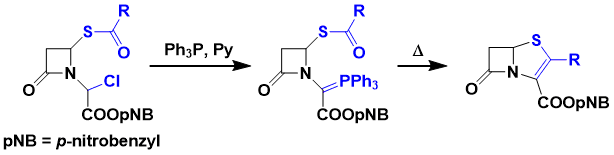
Phosphorus ylides do not generally react with esters. However, they react with esters intramolecularly to give enol ethers.
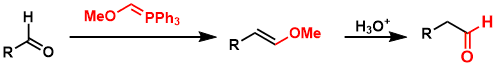
Methoxymethylenetriphenylphosphine, prepared from MOMCl and PPh3, is useful as a one-carbon elongation unit for aldehydes/ketones.[1]
Wittig reaction using catalytic amounts of phosphorus reagents is possible.[2]。
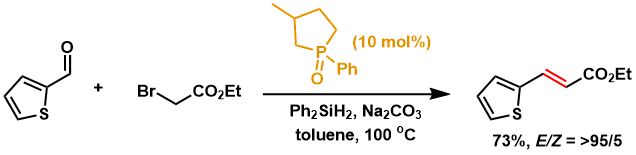
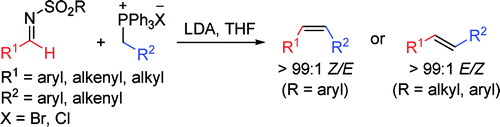
The use of sulfonyl imine instead of carbonyls enables controls of olefine stereoisomerism based on nitrogen substitutents[3]。
-
Experimental Procedur
An exo-olefin from a ketone[4]
Methyltriphenylphosphonium bromide (7.84 g, 21.6 mmol) is suspended in anhydrous THF (10 mL), cooled to -78°C, and n-butyl lithium (20.7 mmol) is added. The reaction mixture is raised to 0°C and stirred for 1 hour. Cool again to -78°C and slowly add a THF solution (90 mL) of ketone (6.3 g, 19.77 mmol). Then, stir at 0°C for 5 hours. An aqueous solution of ammonium chloride is added at 0°C, and the aqueous layer is extracted with diethyl ether. The organic phases are combined, washed with brine, and dried over anhydrous sodium sulfate. After filtration, the solvent is removed under reduced pressure, and the residue is purified by flash column chromatography (hexane/ethyl acetate = 10/1) to give the desired product (5.25 g, 84% yield).
-
Experimental Tips
Methyltriphenylphosphonium bromide is ionic and often contains water. Use it after drying under reduced pressure.
-
References
- Levins, S. G. J. Am. Chem. Soc. 1958, 80, 6150. DOI: 10.1021/ja01555a068
- (a) O’Brien, C. J.; Tellez, J. L.; Nixon, Z. S.; Kang, L. J.; Carter, A. L.; Kunkel, S. R.; Przeworski, K. C.; Chass, C. A. Angew. Chem. Int. Ed. 2008, 48, 6836 DOI: 10.1002/anie.200902525 (b) O’Brien, C. J. et al. Chem. Eur. J. 2013, 19, 15281. DOI: 10.1002/chem.201301444
- Dong, D.-J. .; Li, H.-H. ; Tian, S.-K. J. Am. Chem. Soc. 2010, 132, 5018. DOI: 10.1021/ja910238f
- White, J. D.; Wang, G.; Quaranta, L. Org. Lett. 2003, 5, 4983. DOI: 10.1021/ol035939e
-
Related Reactions
- Barton Kellogg Reaction
- Julia-Kocienski Olefination
- Crabbe Allene Synthesis
- HWE Reaction
- Julia-Lythgoe Olefination
- McMurry Coupling
- Petasis-Ferrier Rearrangement
- Ramberg Backlund Rearreangement
- Seyferth-Gilbert Alkyne Synthesis
- Takeda Olefination
- Takai-Uchiymoto Olefination
- Takai-Lombardo Reaction
- Tebbe Reagent
-
Related Books
[amazonjs asin=”352730634X” locale=”JP” title=”Modern Carbonyl Olefination: Methods and Applications”]
-
External Links
- Wittig Reaction (organic-chemistry.org)
- Mechanism of Wittig Reaction
- Wittig Reaction(Wikipedia)
- Georg Wittig (Wikipedia)
- Georg Wittig-Curriculum Vitae
- Japanese version of this article