Lee, M.K.; Rai, P.; Williams, J.; Twieg, R. J.; Moerner, W. E. J. Am. Chem. Soc. 2014, 136, 14003–14006
DOI: 10.1021/ja508028h
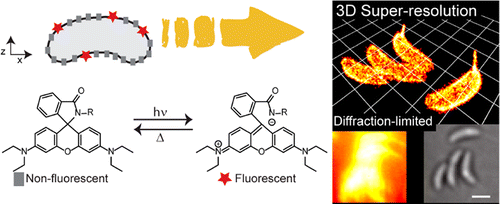
Precise imaging of the cell surface of fluorescently labeled bacteria requires super-resolution methods because the size-scale of these cells is on the order of the diffraction limit. In this work, we present a photocontrollable small-molecule rhodamine spirolactam emitter suitable for non-toxic and specific labeling of the outer surface of cells for three-dimensional (3D) super- resolution (SR) imaging. Conventional rhodamine spiro- lactams photoswitch to the emitting form with UV light; however, these wavelengths can damage cells. We extended photoswitching to visible wavelengths >400 nm by iterative synthesis and spectroscopic characterization to optimize the substitution on the spirolactam. Further, an N-hydroxysuccinimide-functionalized derivative enabled covalent labeling of amines on the surface of live Caulobacter crescentus cells. Resulting 3D SR reconstruc- tions of the labeled cell surface reveal uniform and specific sampling with thousands of localizations per cell and excellent localization precision in x, y, and z. The distribution of cell stalk lengths (a sub-diffraction-sized cellular structure) was quantified for a mixed population of cells. Pulse-chase experiments identified sites of cell surface growth. Covalent labeling with the optimized rhodamine spirolactam label provides a general strategy to study the surfaces of living cells with high specificity and resolution down to 10−20 nm.
Introduction
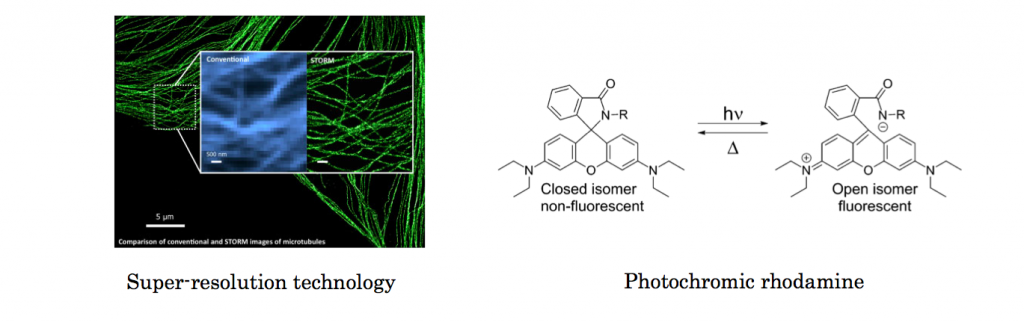
So-called super resolution has been indispensable technology in microscopy and bio-related field.[1] Although various nanoscopy method such as STED, PALM and RESOLFT have already been practiced in many field, the labeling dye is still limited. Photochromic rhodamine is promising dye for both PALM-type and STED-type super resolution technique. However, almost all rhodamine including relative photochromic derivatives suffer from its inability to be photoswitched from non-fluorescent state to fluorescent state by alternative radiation of visible light.[2]
Here, Moerner, who is a winner of Nobel prize in Chemistry 2014, in University of Stanford and his co-worker improved the photophysical properties of classical phorochromic rhodamine and satisfied requirements of super resolution microscopy.
Molecular design
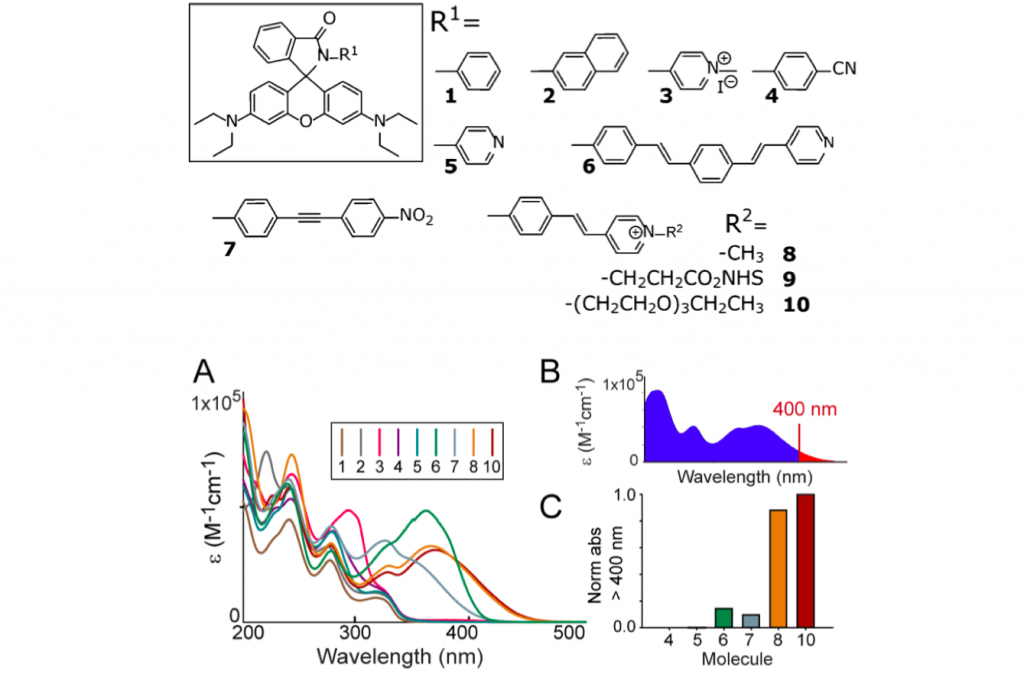
For photochromic rhodamine to be excitable and interconverted between binary state, authors introduced various aromatic groups to lactone moiety. As a result, the introduction of positive charged stilbazolium made the absorption region of open ring form, none fluorescent state, overlaps 405 nm, thus satisfying one of the most tough but important requirement.
3D super resolution image
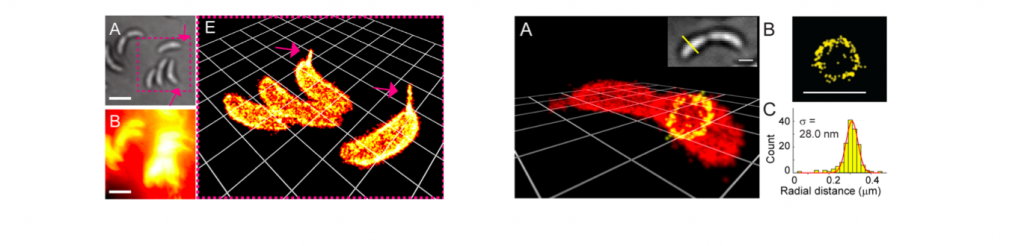
N-hydroxysuccinimide, the most widely used reactive bio-tag, was introduced to the synthesized rhondamine. After stained live Caulobacter crescentus bacterial cells, it was revealed that the dyes localized and bound the surface of the cell. Then, PALM method was used to reconstruct the three dimensional image from discrete single molecule image of photochromic rhodamine.
Reference
(1)“Imaging Intracellular Fluorescent Proteins at Nanometer Resolution”
Betzig, E.; Patterson, G. H.; Sougrat, R.; Lindwasser, O. W.; Olenych, S.; Bonifacino, J. S.; Davidson, M. W.; Lippincott-Schwartz, J.; Hess, H. F. Science 2006, 313, 1642−1645. DOI:10.1126/science.1127344
(2)”Photochromic Rhodamines Provide Nanoscopy with OpticalSectioning”
Fölling, J.; Belov, V.; Kunetsky, R.; Medda, R.; Schönle, A.; Egner, A.; Bossi, M.; Hell, S. W. Angew. Chem., Int. Ed. 2007, 46, 6266−6270. DOI: 10.1002/anie.200702167