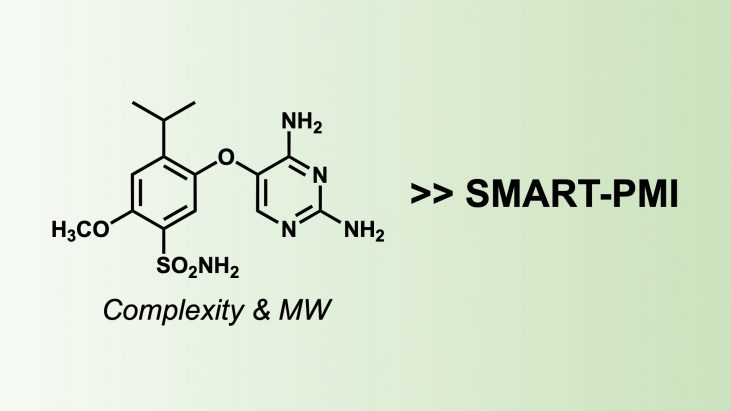
In the industrial production of pharmaceuticals, there is a strong demand to realize synthetic routes that are economical to produce and consider their environmental impact. From the perspective of green and sustainable chemistry, Process Mass Intensity (PMI) is an important indicator to evaluate the impact of a given synthetic route on raw materials, cost, and sustainability. Calculating and optimizing the PMI of an overall or specific step is becoming a common practice in the pharmaceutical industry worldwide, especially in process chemistry teams.
In 2022, Sherer et al. at Merck (MSD) developed SMART-PMI (in-Silico MSD Aspirational Research Tool) to predict PMI from the molecular structure of a synthetic target alone.[1] This method uses only the two-dimensional chemical structure to obtain predictions of PMI from the molecular complexity (Complexity) and molecular weight (MW) of the molecule.
SMART-PMI = (0.13 x MW) + (177 x Complexity) – 252
Each coefficient and constant used to predict the SMART PMI are calculated by a machine learning model reproducing the in-house PMI data obtained within the MSD by Complexity and MW. The complexity of the numerator “Complexity” is calculated by a method previously developed by the authors [2] (the code is available on GitHub [3]).
How to use SMART-PMI
From a process chemistry standpoint, since SMART-PMI is predicted only from the structure, it can be used to indicate how much a bad (large) PMI obtained by the medicinal route can be improved (reduced) by process chemistry. For example, if the actual PMI divided by the SMART-PMI is around 1 (0.9~1.1), it can be evaluated as “Successful” because the process has been optimized to the same level as previously developed processes. In the same way, [Actual PMI]/[SMART-PMI] = 0.5~0.9 can be evaluated as “Exceptional.” “Aspirational” if it can be reduced to less than 0.5. From the standpoint of molecular designers such as medicinal chemists, when multiple candidate compounds can be selected, it may be easier to avoid risks in the manufacturing process by selecting the one with the smaller SMART-PMI.
An Example
Gefapixant (MK-7264)[1]
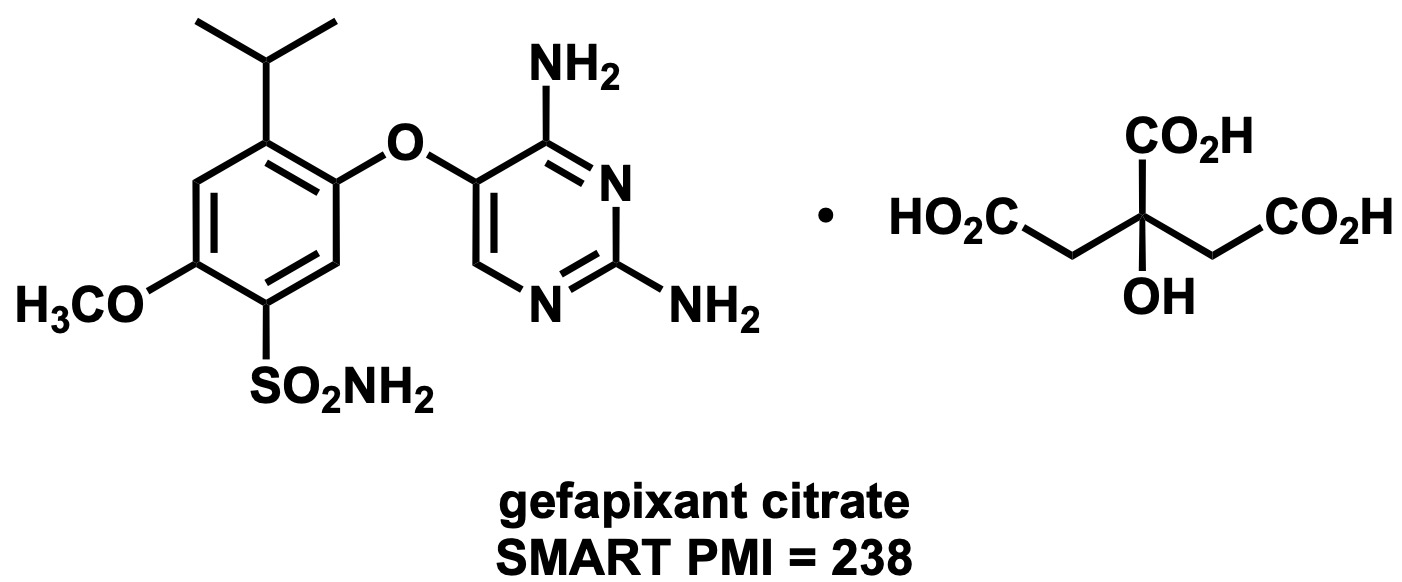 Complexity = 2.4 (without citrate); MW = 353 (without citrate)
Complexity = 2.4 (without citrate); MW = 353 (without citrate)
SMART-PMI = 218 (without citrate) + 20 (citrate) = 238 (including citrate)
PMI Successful = 216–259; PMI Exceptional = 129–215; PMI Aspirational = <129
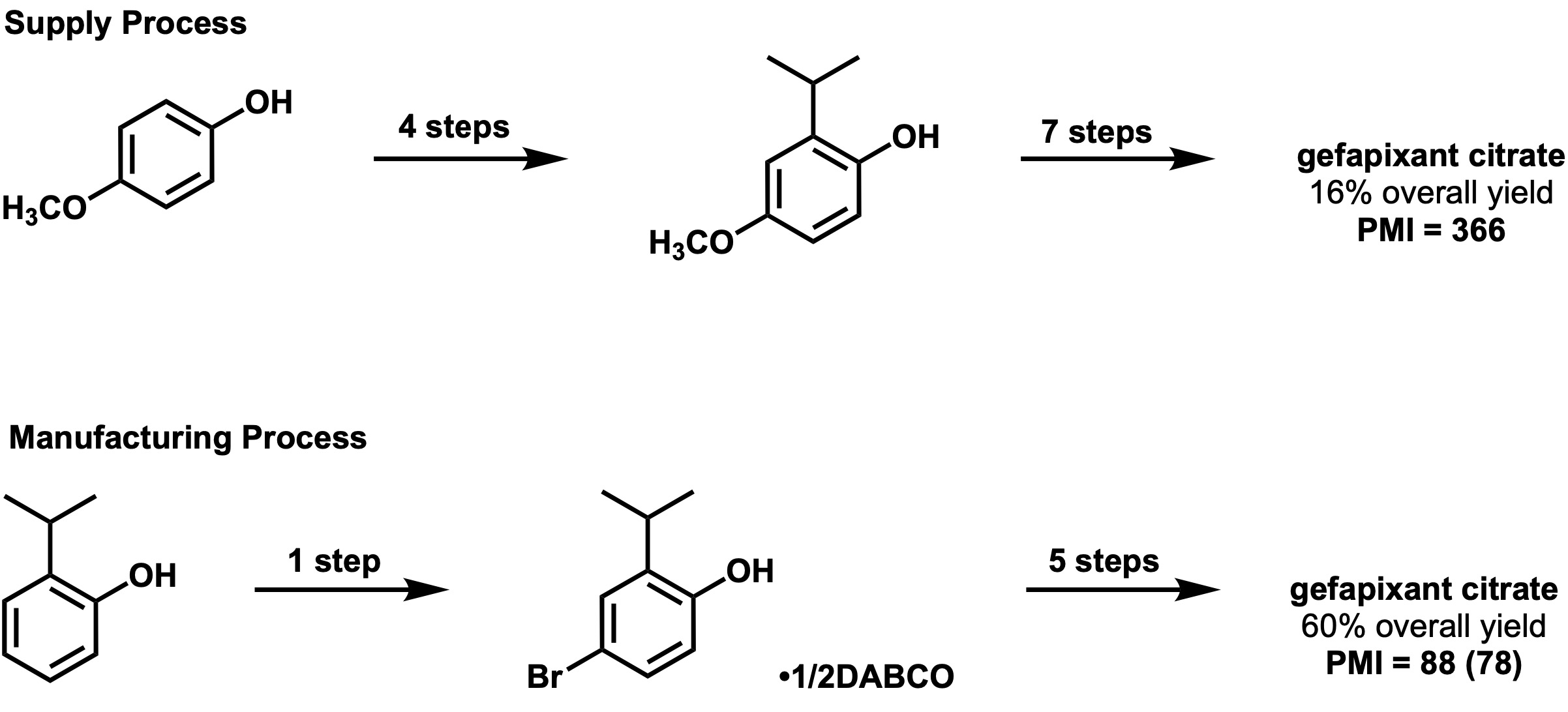
Supply process PMI = 366 … Larger than SMART-PMI (238) and thus can be improved.
Manufacturing route PMI = 88[1] (78 in the original article[4]) … Evaluated as an “Aspirational” route.
References
- Sherer, E. C.; Bagchi, A.; Kosjek, B.; Maloney, K. M.; Peng, Z.; Robaire, S. A.; Sheridan, R. P.; Metwally, E.; Campeau, L.-C. Driving Aspirational Process Mass Intensity Using Simple Structure-Based Prediction. Org. Process Res. Dev. 2022, 26, 1405-1410. DOI: 10.1021/acs.oprd.1c00477
- Sheridan, R. P.; Zorn, N.; Sherer, E. C.; Campeau, L.-C.; Chang, C. Z.; Cumming, J.; Maddess, M. L.; Nantermet, P. G.; Sinz, C. J.; O’Shea, P. D. Modeling a Crowdsourced Definition of Molecular Complexity. Journal of Chemical Information and Modeling 2014, 54, 1604-1616. DOI: 10.1021/ci5001778
- https://github.com/Merck/compoundcomplexity
- Ren, H.; Maloney, K. M.; Basu, K.; Di Maso, M. J.; Humphrey, G. R.; Peng, F.; Desmond, R.; Otte, D. A. L.; Alwedi, E.; Liu, W.; et al. Development of a Green and Sustainable Manufacturing Process for Gefapixant Citrate (MK-7264) Part 1: Introduction and Process Overview. Org. Process Res. Dev. 2020, 24, 2445-2452. DOI: 10.1021/acs.oprd.0c00248