This post introduces NMR basics, focusing on the analysis and interpretation of the 1-D 1H and 13C NMR spectra. An overview of NMR basics, including principles, is available on the JEOL website.
Information available from NMR charts
Cited from the JEOL website.
Three major aspects available from NMR charts after the Fourier transformation are:
- Chemical shift (δ)
- Spin-spin coupling constant (J)
- The area under the peak (integral)
1. Chemical shift (δ)
A nucleus is surrounded by a cloud of electrons. When negatively charged electrons circulate around the nucleus in an external magnetic field B0, they generate an induced magnetic field B‘ in the opposite direction. The effective magnetic field B experienced by the nucleus is reduced by that amount (B‘):
B = B0 – B‘
This apparent decrease in a magnetic field is called the “shielding effect.” The shielding is strong when (1) the electron density around the nucleus is large and (2) the aromatic ring current effect is active.
The strength of the magnetic field experienced by the nucleus differs depending on their environment. As a result, the resonance frequencies are “shifted” from the standard. This “deviation” is called the chemical shift. In other words, chemical shifts provide information about the environment where the nuclei are located.
The chemical shift value in a normal 1-D 1H and 13C NMR measurement is shown using a ppm scale as a relative value with that for the methyl group of tetramethylsilane (TMS, reference, δ = 0). Chemical shift values for substructures are known (see figure below).
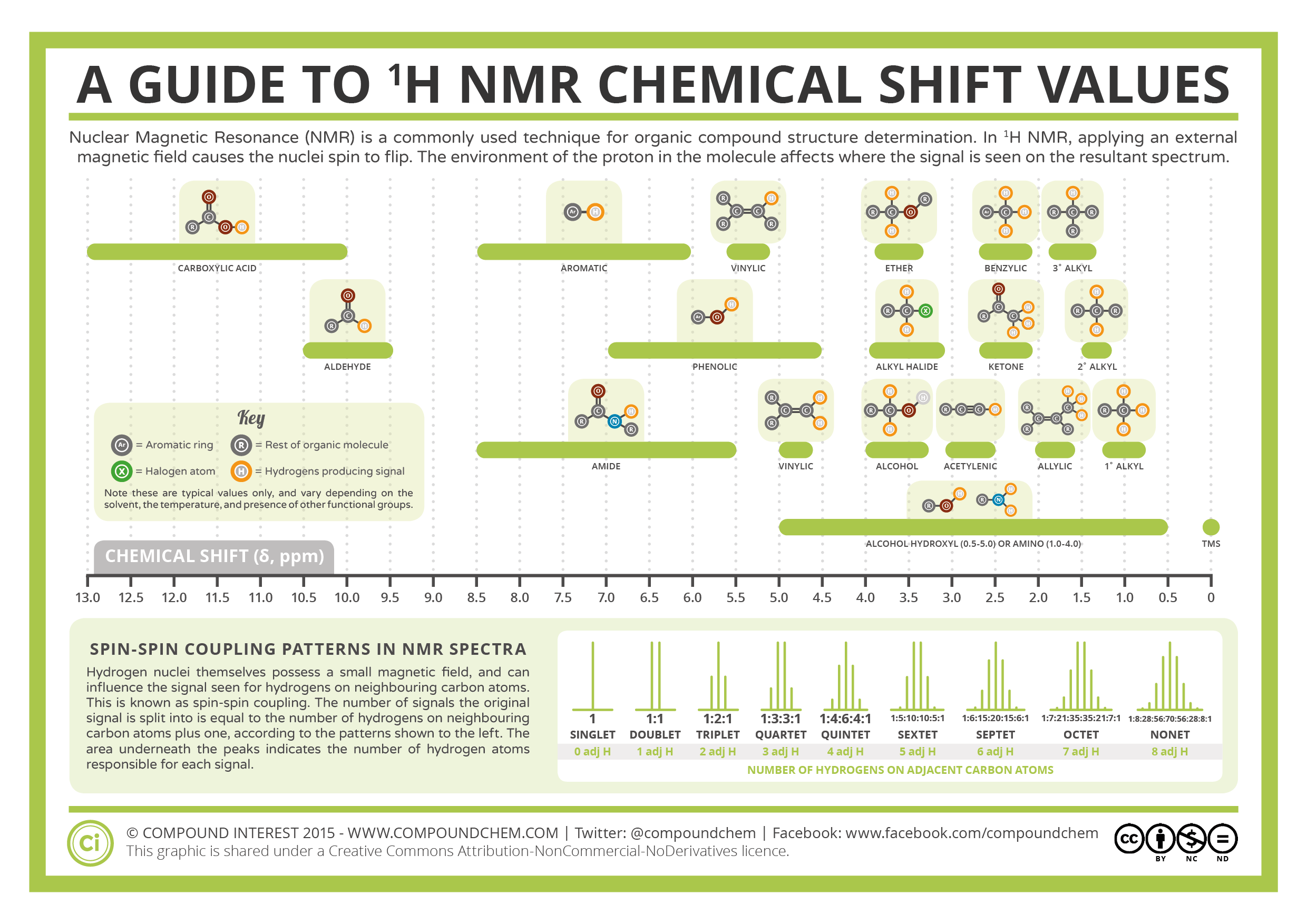
Click to enlarge. Cited from this page
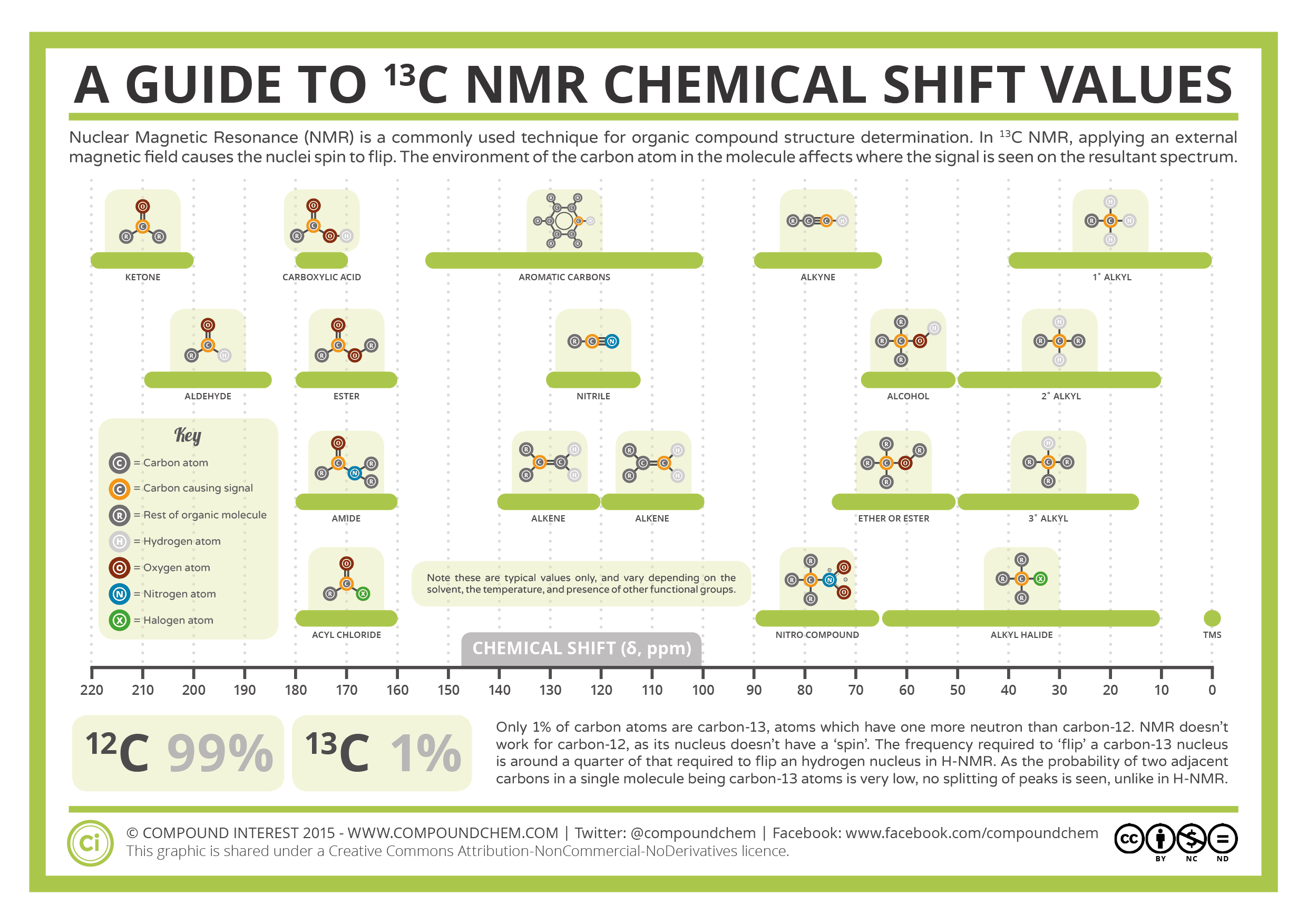
Click to enlarge. Cited from this page
2. Spin-Spin coupling constant (J)
When chemically inequivalent nuclei are in close proximity to each other, they magnetically interact with each other, causing energy level splitting. This phenomenon is called coupling.
On a 1H-NMR chart, when n equivalent protons Hb exist next to proton Ha, the peak of Ha is observed to split into (n+1) peaks. Each peak is denoted by the following symbols depending on the number of peaks.
Single line: singlet (singlet, s)
Doublet (doublet, d)
Triple line: triplet (triplet, t)
Four lines: Quartet (q)
Broad line: broad (br)
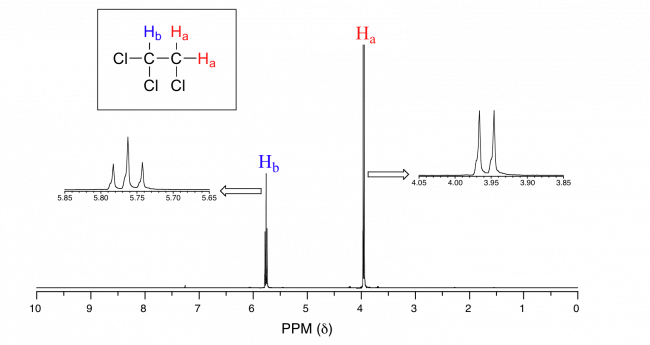
In the following example, there are two chemically equivalent Ha peaks next to Hb. Therefore, the peak of Hb is a triplet (t) divided into 2+1=3 lines. From the point of view of Ha, there is only one neighboring Hb, so the peak of Ha is a doublet (d) with 1+1=2 peaks.
The distance between coupled peaks can be converted to the spin coupling constant (J-value). The J-value is an indicator of which nuclides are in close proximity.
J (Hz) = measurement frequency (Hz) x chemical shift difference (Δδ, ppm)
For example, if 1H-NMR is measured on a 500 MHz instrument and the chemical shift difference of the peaks of a signal is Δδ = 0.015 ppm, the J value can be calculated as (500 x 106) x (0.015 x 10-6) = 7.5 Hz.
The J values are normalized with the magnet strength (measurement frequency), so the values do not depend on the magnet strength (NMR machines).
This coupling appears between hetero nuclei. So, carbon (13C) also couples with protons (1H). To simplify the 13C NMR spectra by removing coupling information with protons (and to increase sensitivity), typical 13C NMR is measured decoupled with protons (denoted as 13C{1H} NMR).
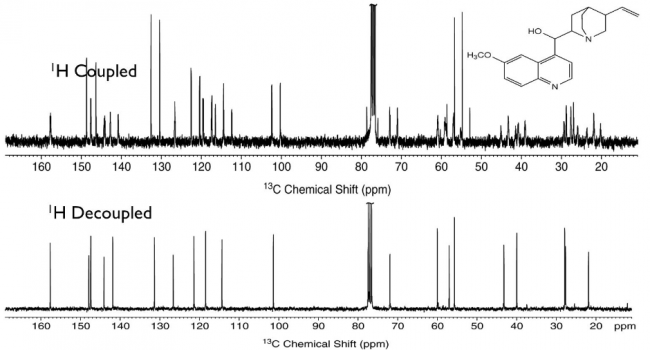
13C-NMR Spectra with and without 1H-decoupling (Cited from this slide)
13C-13C coupling is not observed because of the low natural abundance of 13C over 12C.
3. Area under the peak (integral)
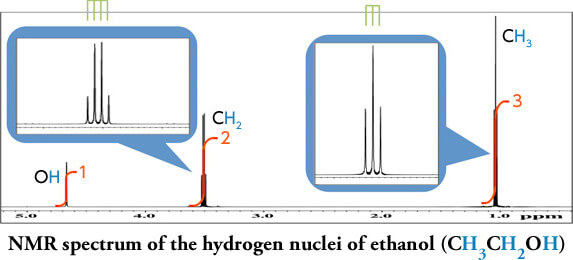
The area value of each peak obtained after Fourier transform corresponds to the ratio of the nuclei. The 1H NMR is quantitative, and the integral ratio can be used to determine the ratio of protons in the same environment.

Cited from the JEOL website.
Note that the integrals reflect the ratio, not the number of nuclei. Also acidic protons (e.g., OH) can easily exchange with deuterated solvents (such as CD3OD) and become invisible.
13C{1H} NMR integrals are not used for quantification because of the lack of quantitativity (reflecting the efficiency of decoupling).
Sample Preparation
Dissolve a sample compound (several mg for 1H-NMR) in NMR measurement solvent (about 0.4 mL). Place this in an NMR tube (outer diameter of approx. 5 mm) and cap it. The sample must be completely dissolved and in a clear solution. If the sample is suspended, good-quality data will not be obtained. Hight of the solution is also important for clear spectra.
To measure 1H-NMR, use a heavy solvent in which the hydrogen (H) in the solvent is replaced by deuterium (D). This is because the 1H peak of the sample compound will be buried in the chart if a non-deuterated solvent that contains many protons is used. The deuterated solvents are also used for “Lock” in NMR using deuterium nuclei.
Deuterated chloroform (CDCl3) is most frequently used for routine measurements in organic chemistry due to the following reasons:
- Only one deuterium is contained, making it inexpensive to produce.
- The moderately low boiling point allows the sample to be recovered after measurement by removing the solvent.
- Many organic compounds dissolve in CDCl3 and do not react with CDCl3.
- CHCl3 is a frequent impurity, but its singlet signal does not overlap with most of the sample peaks.