Alexey N. Butkevich, Grazv̌ydas Lukinavicǐus, Elisa D’Este, and Stefan W. Hell. J. Am. Chem. Soc., 2017, 139, 12378.
Abstract
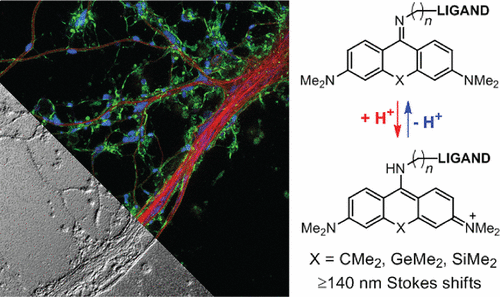
Abstract: We designed cell-permeant red-emittingfluorescent dye labels with >140 nm Stokes shifts based on 9-iminoanthrone, 9-imino-10-silaxanthone, and 9- imino-10-germaxanthone fluorophores. The corresponding probes selectively targeting mitochondria, lysosomes, and F-actin demonstrate low toxicity and enable stimulated emission depletion (STED) nanoscopy in neurons, humanfibroblasts, U2OS, and HeLa cells. In combination with known small Stokes shift dyes, our probes allow live-cell three-color STED nanoscopy of endogenous targets on popular setups with 775 nm STED wavelength.
Introduction
Multicolor super-resolution fluorescence microscopy is a valuable method for observing interactions between intracellular structures and biomolecules. A straightforward way to achieve multi-color imaging is to introduce a third channel on the widely available nanoscopes with 775 nm STED wavelength relied on using large Stokes shift (LSS) labels. However, LSS dyes for live-cell labeling have still been missing. In this paper, S. W. Hell, and his co-workers developed of LSS fluorophores capable of penetrating intact plasma membranes of living cells. Besides, they should be compatible with the popular 775 nm STED wavelength. Following the recent report by the Klań group [1] and an earlier work of Wu and Burgess,[2] they had identified 9-aminopyronin scaffold as a promising LSS analog of rhodamine.
Details
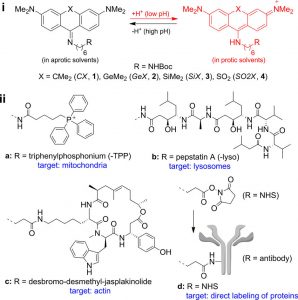
Figure 1 shows Chemical structures of LSS probes. (i) Protonation− deprotonation behavior responsible for the environment sensitivity of the dyes 1−4. (ii) Ligands a−d for labeling of intracellular structures.
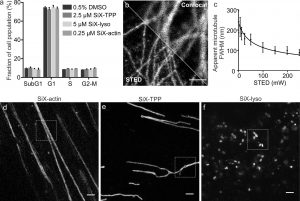
Figure 2 shows the performance of SiX probes targeted to different cellular organelles. (a) Percentage of cells with different measured DNA content after 24 h treatment with indicated probes vs DMSO vehicle, corresponding to cell cycle phases: G1, S, G2, and M. Cells containing less DNA than haploid (SubG1) are considered non-viable. (b) Confocal and STED images of fixed human fibroblasts stained with primary mouse anti-α-tubulin and SiX-labeled secondary sheep anti-mouse antibodies. (c) Dependence of micro- tubule apparent thickness FWHM on the de-excitation power applied. (d−f) STED images of living human fibroblasts stained with (d) 0.25 μM SiX-actin (3c), (e) 2 μM SiX-TPP (3a), and (f) 3 μM SiX-lyso (3b)
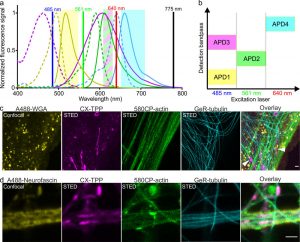
Figure 3 shows the result of four-color microscopy with CX-TPP probe. (a) Normalized absorption and emission spectra of the dyes Alexa Fluor 488 (yellow), CX (magenta), 580CP (green), and GeR (cyan) utilized for simultaneous multicolor imaging. De-excitation laser (black line) is set at 775 nm. (b) Excitation/detection scheme used for four-color imaging. (c) Four-color image of living human fibroblasts stained with 1 μM 580CP-actin, 2 μM GeR-tubulin, 2 μM CX-TPP (1a), and 5 μg/mL Alexa Fluor 488-WGA conjugate. (d) Four-color image of living rat hippocampal neurons (16 DIV) stained with 1 μM 580CP-actin, 2 μM GeR-tubulin, 0.5 μM CX-TPP.
Reference
[1]”Small-Molecule Fluorophores with Large Stokes Shifts: 9‐Iminopyronin Analogues as Clickable Tags”
Horvath, P.; Sebej, P.; Solomek, T.; Klan, P. J. Org. Chem. 2015, 80, 1299−311.
[2]”Fluorescent Amino- and Thiopyronin Dyes”
Wu, L.; Burgess, K. Org. Lett. 2008, 10, 1779−82.
DOI: 10.1021/ol800526s