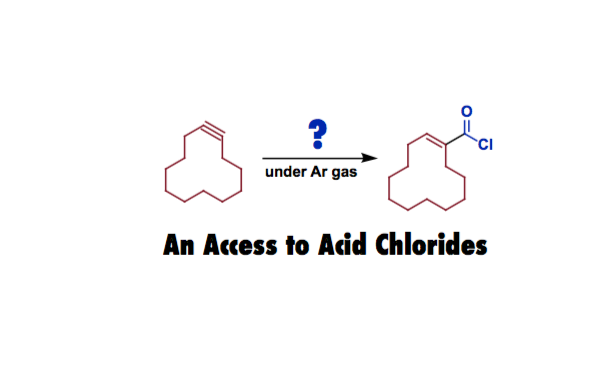
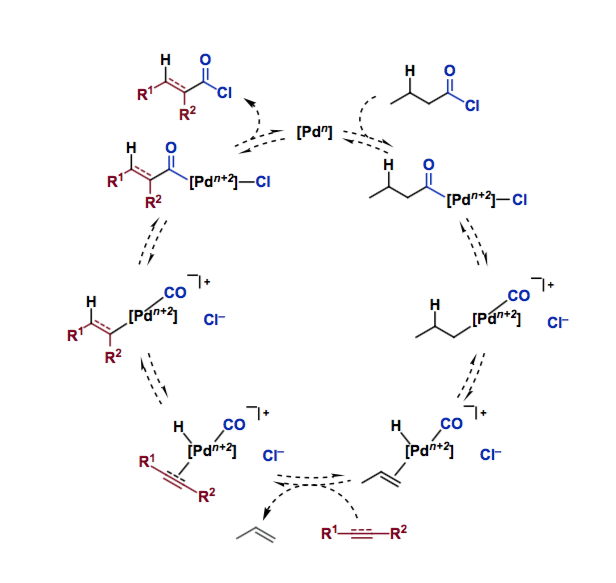
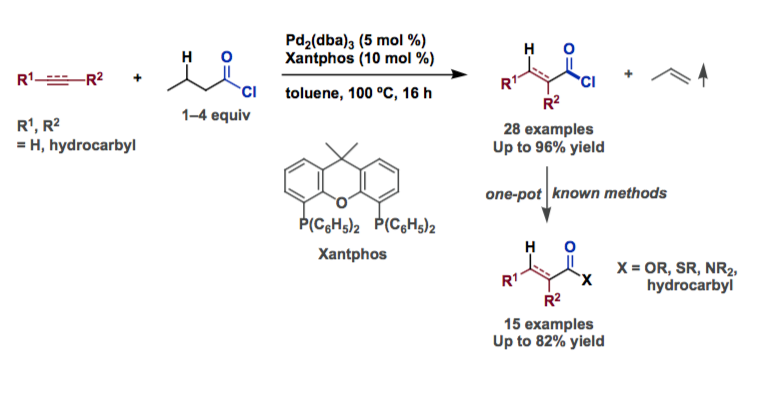
Morandi at Max-Planck-Institut für Kohlenforschung developed an exchange reaction for producing functionalized acid chlorides.
“CO- and HCl-free synthesis of acid chlorides from unsaturated hydrocarbons via shuttle catalysis” Fang, X.; Cacherat, B.; Morandi, B.* Nat. Chem. 2017, 9, 1105–1109. DOI: 10.1038/NCHEM.2798
Introduced by R.N.
Issue
Acid chlorides are widely used compounds in organic synthesis. A traditional and powerful method to access these compounds is the Reppe-type carbonylation, in which olefins or alkynes react with CO and HCl. But both CO and HCl are not easy to handle due to their toxic and corrosive nature. So an alternative, convenient method for the synthesis of acid chlorides has been demanded.
Approach
Morandi and coworkers applied their shuttle-catalysis approach [1,2] to the CO- and HCl-free hydrochlorocarbonylation of alkynes and alkenes. They used inexpensive butyryl chloride as a CO and HCl donor in order to convert a broad range of functionalized unsaturated hydrocarbons into the corresponding acid chlorides. Their scheme includes oxidative addition, CO de-insertion, beta-hydride elimination, ligand exchange, and reverse reactions of those processes.
Result
The versatility of this process is demonstrated in a broad set of one-pot reactions that can transform unsaturated substrates into several carboxylic acid or ketone derivatives.
Author Information
PI Prof. Bill Morandi (Max-Planck-Institut für Kohlenforschung)
References
- Fang, X.; Yu, P.; Morandi, B. Science 2016, 351, 832–836. DOI: 10.1126/science.aae0427
- Bhawal, B. N.; Morandi, B. ACS Catal. 2016, 6, 7528−7535. DOI: 10.1021/acscatal.6b02333
Related Books
[amazonjs asin=”B014P9HU5Y” locale=”JP” title=”Advanced Organic Chemistry: Part A: Structure and Mechanisms: Structure and Mechanisms Pt. A”]
[amazonjs asin=”0387683542″ locale=”JP” title=”Advanced Organic Chemistry: Part B: Reaction and Synthesis (Advanced Organic Chemistry / Part B: Reactions and Synthesis)”]
[amazonjs asin=”B00JDCP7S6″ locale=”JP” title=”The Organometallic Chemistry of the Transition Metals”]
[amazonjs asin=”0198559178″ locale=”JP” title=”Applied Organometallic Chemistry and Catalysis (Oxford Chemistry Primers)”]