Kraskouskaya, D.; Bancerz, M.; Soor, H. S.; Gardiner, J. E.; Gunning, P. T. Am. Chem. Soc., 2014, 136, 1234–1237
DOI: 10.1021/ja411492k
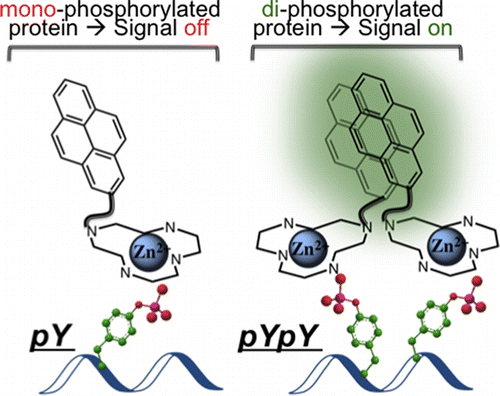
ABSTRACT: Protein phosphorylation is a ubiquitous post-translational modification, which often acts as a switch to proteins’ activation and is frequently perturbed in diseases. Although many general phospho-protein detec- tion tools are available, none of them offers information about the relative spatial arrangement of phosphorylated residues. Specifically, proximally phosphorylated residues are hallmarks of certain activated disease-relevant proteins. We herein report the first turn-on fluorescent sensor for the selective detection of proximally phosphorylated protein sites, suitable for application in both aqueous solutions and polyacrylamide gels.
Introduction
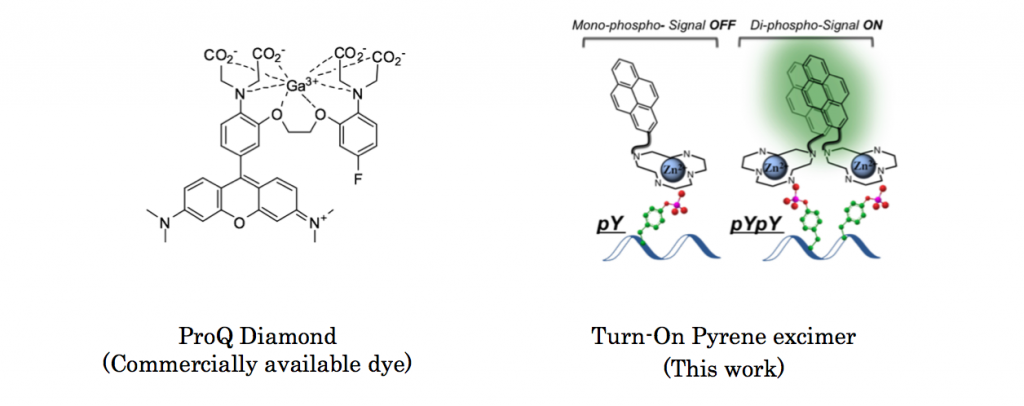
Protein phosphorylation is a reaction where namely protein is phosphorylated so that the subsequent reaction or other bio-related events can smoothly proceed. Although several commercial reagents capable of detecting phosphorylated residues is already available,[1] these reagents do not provide more detailed information about phosphorylated residues. Here, Gunning group in University of Toronto, for the first time, achieved fluorescently detecting proximal phosphorylated residues of protein using classical pyrene excimer formation strategy.[2]
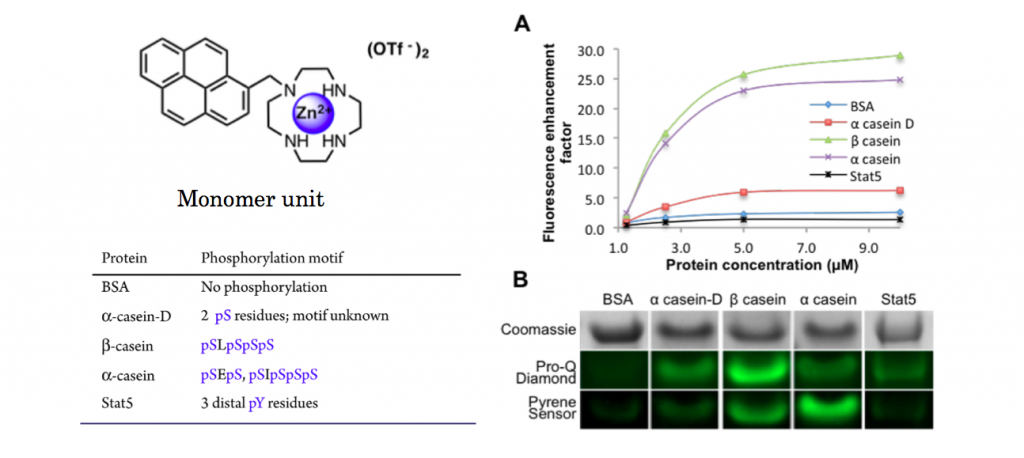
Selectivity to phosphorylated residues
To see the selectivity of synthesized pyrene dye, titillation experiments to 5 proteins were carried out. As shown below, only αcasein and βcasein which has diphosphorylated residues exhibited dramatic increases in fluorescence intensity.
Reference
[1]“Mapping phosphoproteins in Mycoplasma genitalium and Mycoplasma pneumoniae”
Su, H.-C.; Hutchison, C. A.; Giddings, M. C. BMC Microbiol.2007, 7, 63. DOI:10.1186/1471-2180-7-63
[2]“Real-Time Fluorometric Assay for Acetylcholinesterase Activity and Inhibitor Screening through the Pyrene Probe Monomer–Excimer Transition”
Chen, J.; Liao, D.; Wang, Y.; Zhou, H.; Li, W.; Yu, C. Org. Lett. 2013, 15, 2132. DOI: 10.1021/ol400619t