Yamaguchi, T.; Asanuma, M.; Nakanishi, S.;Saito Y.;,Okazaki, M.; Dodoab, K. Sodeoka, M. Chem. Sci., 2014, Advance Article
DOI: 10.1039/C3SC52704B
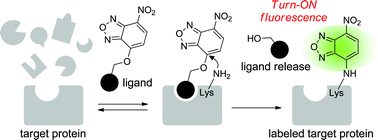
Affinity labeling has become a powerful tool to identify target proteins, as well as to visualize/characterize target functions in living cells. However, although various functional groups have been utilized for affinity labeling, many of them have disadvantages such as complex structure and large size. To address this problem, we designed a simple chemical probe bearing a small bifunctional O-NBD unit (NBD: nitrobenzoxadiazole). Model ligand–protein experiments showed that the O-NBD unit has excellent characteristics for target-specific labeling even in the presence of a large excess of non-target proteins. Moreover, attachment of the O-NBD unit toN,N-dialkyl-2-phenylindol-3-ylglyoxylamides (PIGAs), which are recently developed translocator protein (TSPO) ligands, enabled us to visualize mitochondria expressing TSPO in living cells by means of “turn-ON” fluorescence. Two-dimensional PAGE analysis of the labeled mouse kidney mitochondria strongly suggested that our synthetic probe selectively modified a partner protein of TSPO, voltage-dependent anion channel (VDAC).
Prof. Sodeoka‘s laboratory focuses on the following research based on synthetic organic chemistry: (1) development of new reactions and methodologies for the efficient synthesis of bioactive molecules, (2) design and synthesis of molecules with unique biological activities, (3) biological research using unique molecules as biological probes.
[amazonjs asin=”3527323309″ locale=”US” title=”Chemical Biology”][amazonjs asin=”1118101189″ locale=”US” title=”Chemical Biology: Approaches to Drug Discovery and Development to Targeting Disease”]