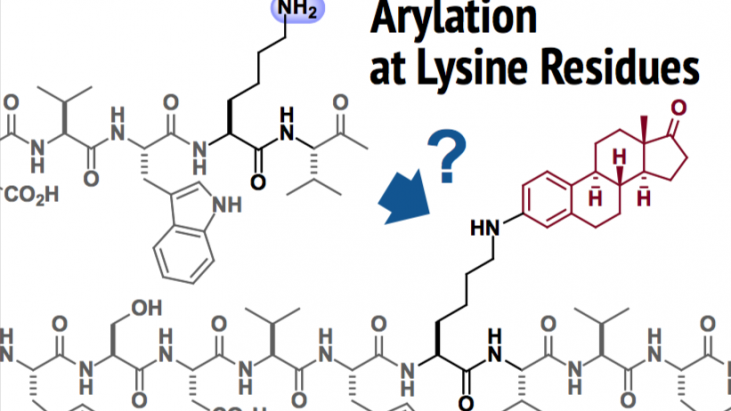
Pentelute and Buchwald et al. at MIT developed a method for chemoselective functionalization of peptides.
“Palladium-Mediated Arylation of Lysine in Unprotected Peptides”
Lee, H.-G.; Lautrette, G.; Pentelute B.L.; Buchwald, S.L. Angew. Chem. Int. Ed. 2017, 56, 3177–3181. DOI: 10.1002/anie.201611202
Introduced by H.O.
Issue
Connecting functional molecules to peptides has become an important technique in the field of chemical biology and medicinal chemistry [1]. Representative chemicals include affinity tags, chromophores, and pharmaceuticals. The modification of amino groups (NH2) at the lysine residue in peptides is one of the major methods for such transformation (e.g. conjugate addition, addition to ketene, and Schiff base formation). Despite the utility of these protocols, chemoselectivity in the functionalization and stability of the conjugate product needed to be improved.
To address this issue, the authors developed a new technique of lysine conjugation by extending their palladium-mediated arylation chemistry.
Approach
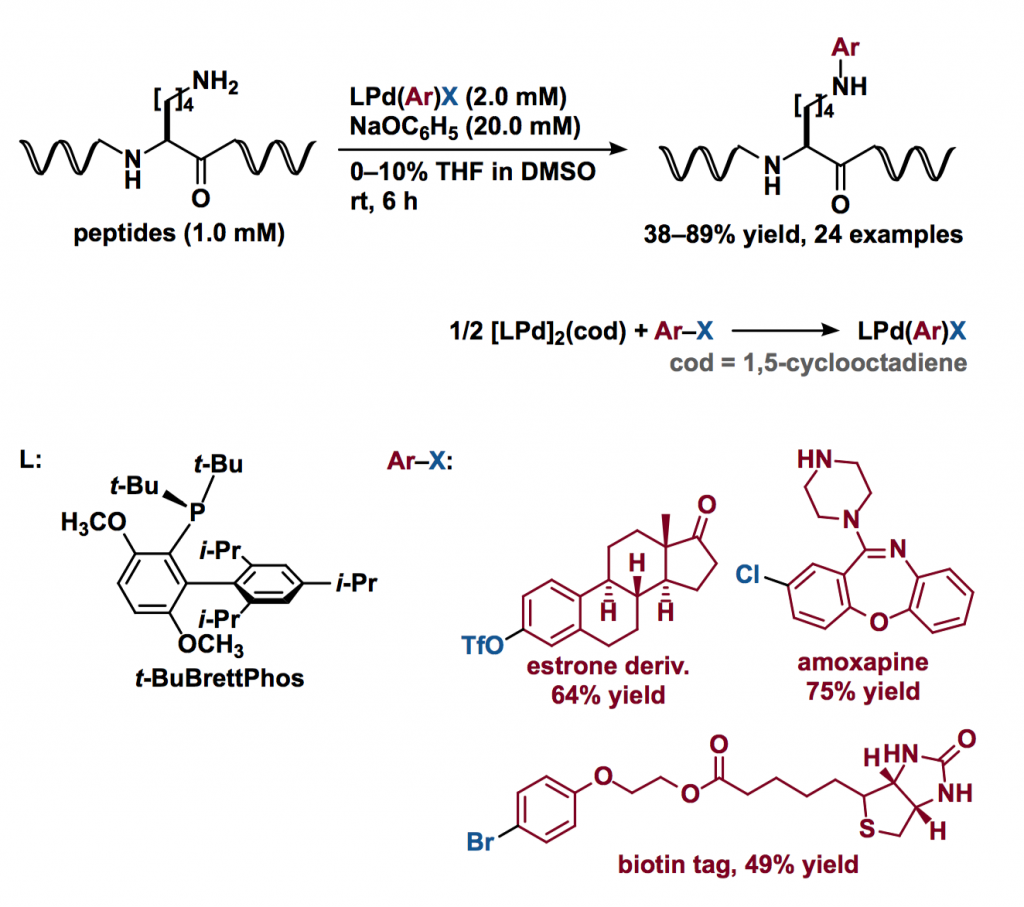
The authors previously developed a palladium-mediated bioconjugation to give cysteine thiol-arylated peptide or protein [2]. Based on this technology, they screened the best ligand and reaction conditions for palladium-mediated arylation of a model peptide containing a lysine residue.
Result
The reaction was best performed with a Pd complex bearing t-BuBrettPhos ligand in the presence of sodium phenoxide at room temperature. By this method, aryl groups derived from natural products, conjugation or affinity tags, chromophores, and complex drug molecules were readily installed at the lysine residue.
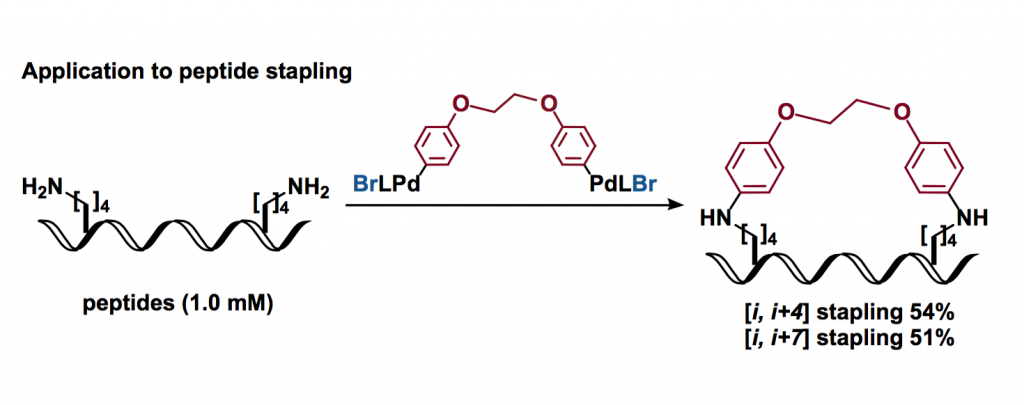
This method could be applied to the peptide stapling [3], forming macrocyclic structures in acceptable yields.
Points and Questions
- Side reactions include ligand arylation.
- Compatible with RSH group at Cys residue? – No. Cys RSH is more reactive than Lys NH2.
Author Information
PI Prof. Bradley L. Pentelute (MIT)
PI Prof. Stephan L. Buchwald (MIT)
References
- Hu, Q.; Berti, F.; Adamo R. Chem. Soc. Rev. 2016, 45, 1691–1719. DOI: 10.1039/C4CS00388H
- Vinogradova, E. V.; Zhang, C.; Spokoyny, A. M.; Pentelute, B. L.; Buchwald, S. L. Nature 2015, 526, 687–691. DOI: 10.1038/nature15739
- Lau, Y.-H.; de Andrade, P.; Wu, Y.; Spring, D. R. Chem. Soc. Rev. 2015, 44, 91–102. DOI: 10.1039/c4cs00246f
Related Books
[amazonjs asin=”0199257388″ locale=”JP” title=”Amino Acid and Peptide Synthesis (Oxford Chemistry Primers, 7)”]
[amazonjs asin=”3527323309″ locale=”JP” title=”Chemical Biology”]
[amazonjs asin=”0815342144″ locale=”JP” title=”Introduction to Bioorganic Chemistry and Chemical Biology”]