- Generality
- Reagent Availabiltiy
- Experimental User Friendliness
- Criteria #4
- Criteria #5
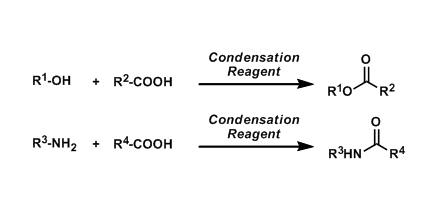
General Characteristics
Esters and amides (peptides) can be obtained by condensing carboxylic acids with alcohols and amines under strongly acidic conditions (Fischer method). However, epimerization at the α-position and side reactions are frequently problematic. A simple solution to this problem is to use a condensation reagent. Various reagents are known so far, but they all proceed basically in the same manner. Each has slightly different characteristics.
Standard Reagents with References
<DCC>
- Sheehan, J. C.; Hess, G. P. J. Am. Chem. Soc. 1955, 77, 1067. DOI: 10.1021/ja01609a099
- Neises, B.; Steglich, W. Angew. Chem. Int. Ed. Engl. 1978, 17, 522. DOI: 10.1002/anie.197805221
<EDC(WSCI)>
- Sheehan, J.; Cruickshank, P.; Boshart, G. J. Org. Chem. 1961, 26, 2525. DOI: 10.1021/jo01351a600
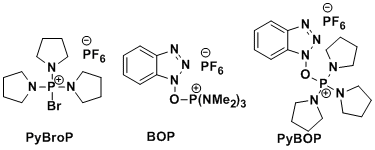
<BOP>
- Castro, B.; Dormoy, J.-R.; Evin, G.; Selve, C. Tetrahedron Lett. 1975, 16, 1219. doi:10.1016/S0040-4039(00)72100-9
<PyBOP>
- Coste, J.; Le-Nguyen, D.; Castro, B. Tetrahedron Lett. 1990, 31, 205. doi:10.1016/S0040-4039(00)94371-5
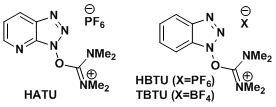
<HATU, HBTU>
- Dourtoglou, V.; Ziegler, J.-C.; Gross, B. Tetrahedron Lett. 1978, 19, 1269. doi:10.1016/0040-4039(78)80103-8
- Knorr, R.; Trzeciak, A.; Bannwarth, W.; Gillessen, D. Tetrahedron Lett. 1989, 30, 1927. doi:10.1016/S0040-4039(00)99616-3
- Carpino, L. A. J. Am. Chem. Soc. 1993, 115, 4397. DOI: 10.1021/ja00063a082
- Carpino, L. A. et al. Angew. Chem. Int. Ed. 2002, 41, 441. [abstract]
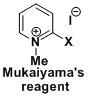
<Mukaiyama reagent>
- Bald, E.; Saigo, K.; Mukaiyama, T. Chem. Lett. 1975, 1163. doi:10.1246/cl.1975.1163
- Mukaiyama, T. Angew. Chem. Int. Ed. Engl. 1979, 18, 707. DOI: 10.1002/anie.197907073
- Huang, H.; Iwasawa, N.; Mukaiyama, T. Chem. Lett. 1984, 1465. doi: 10.1246/cl.1984.1465
<DMT-MM (Kunishima reagent)>
- Kunishima, M., Kawachi, C., Iwasaki, F., Terao, K. Tetrahedron Lett. 1999, 40, 5327. doi:10.1016/S0040-4039(99)00968-5
- Kunishima, M.; Kawachi, C.; Hioki, K.; Terao, k.; Tani, S. Tetrahedron 2001, 57, 1551. doi:10.1016/S0040-4020(00)01137-6
<HOBt additive>
- Carpino, L. A. J. Am. Chem. Soc. 1993, 115, 4397. DOI: 10.1021/ja00063a082
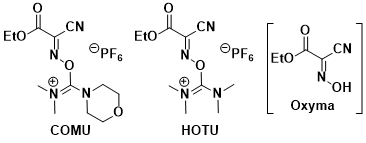
<Oxyma additive>
- Subiros-Funosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Chem. Eur. J. 2009, 15, 9394. DOI: 10.1002/chem.200900614
<COMU>
- El-Faham, A.; Albericio, F. J. Org. Chem. 2008, 73, 2731. DOI: 10.1021/jo702622c
- El-Faham, A.; Funosas, S. R.: Prohens, R.; Albericio, F. Chem. Eur. J. 2009, 15, 9404. DOI: 10.1002/chem.200900615
- Subiros-Funosas, R.; Nieto-Rodriguez, L.; Jensen, K. J.; Albericio, F. J. Pept. Sci. 2013, 19, 408. doi:10.1002/psc.2517
<Review of Peptide Coupling Reagent>
- Han, S.-Y.; Kim, Y.-A. Tetrahedron 2004, 60, 2447. doi:10.1016/j.tet.2004.01.020
- Montalbetti, C. A. G. N.; Falque, V. Tetrahedron 2005, 61, 10827. doi:10.1016/j.tet.2005.08.031
- Valeur, E.; Bradley, M. Chem. Soc. Rev. 2009, DOI: 10.1039/b701677h
- El-Faham, A.; Albericio, F. Chem. Rev. 2011, 111, 6557. DOI: 10.1021/cr100048w
<General Review of Chemical Synthesis of Peptides/Prtoeins>
- Humphrey, J. M.; Chamberlin, A. R. Chem. Rev. 1997, 97, 2243. DOI: 10.1021/cr950005s
- Kent, S. B. H. Chem. Soc. Rev. 2009, 38, 338. DOI: 10.1039/b700141j
- Pattabiraman, V. R.; Bode, J. W. Nature 2011, 480, 471. doi:10.1038/nature10702
Typical Reaction Mechanism
An example of DCC-mediated condensation:
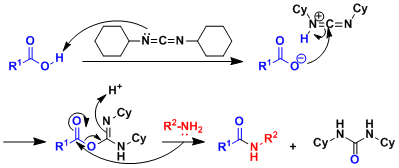
Typical peptide synthesis involves the elongation at N-terminus. Elongation at the C-terminus is problematic because it involves intramolecular cyclization to give azlactone (and racemization; see below figure). The racemization can be suppressed by using nucleophilic reagents such as HOBT, HOAt, and Oxyma (Cf. J. Am. Chem. Soc. 1964, 86, 2918.).
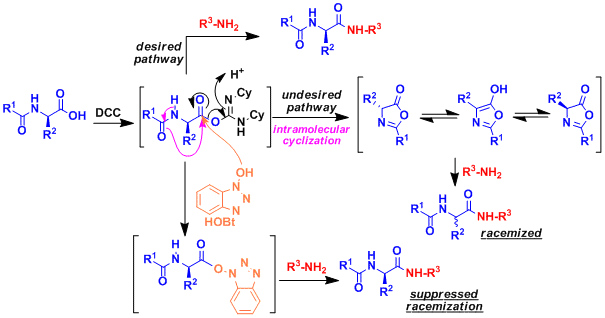
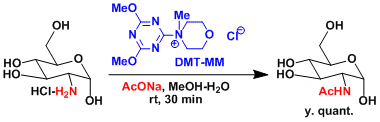
Example
Amide formation with DMT-MM is oparative under protic(aqueous) conditions.[1]
List of Popular reagents
- DCC(dicyclohexylcarbodiimide): Most frequently used and practical. However, its toxicity and the difficulty of removing the crystalline urea byproduct are problematic.
- DIC(diisopropylcarbodiimide): Similar to DCC.
- EDC(1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide; WSCI): Product separation is easy because the urea byproduct is water soluble and easily removed. It’s more expensive than DCC.
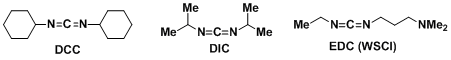
- HATU, HBTU, TATU, TBTU: Hybrid reagents that form HOAt and HOBt in situ. Effective for suppressing epimerization. Expensive. HATU is one of the most reliable reagents for peptide coupling.
- COMU, HOTU: Hybrid reagents that form a nucleophile called Oxyma. COMU performs better than HATU in some cases. Byproducts are water-soluble and easily removed. COMU generates reactive O-acyl intermediates.
- BOP-Cl: It is not produced anymore because of its carcinogenic character.
- PyBOP, BOP, PyBroP: Crystalline solids.
- DPPA(diphenyl phosphoryl azide, Shioiri Reagent): Advantageous over DCC because the byproducts are easily removed.
- DMT-MM(2-Chloro-4,6-dimethoxy-1,3,5-triazine + N-methyl morpholine, Kunishima Reagent): Operative under aqueous or alcoholic conditions. Epimerization is surpressed. Excess reagents and byproducts can be removed by HCl aq wash. Very practical.
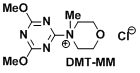
- Mukaiyama Reagent
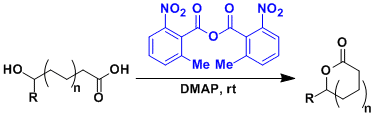
- Corey-Nicolaou Method, Yamaguchi Macrolactonization, Keck Method: for macrolactonization.
- Shiina Macrolactonization: more reactive than Yamaguchi Macrolactonization.
- Mitsuhobu Reaction: Involves stereochemical inversion of alcohols. Useful for various reactions, including macrolactonization.
- Mukaiyama Quinone method: Operative with tertiary alcohols, involves stereochemical inversion of alcohols.
Reference
[1] Kunishima, M., Kawachi, C., Iwasaki, F., Terao, K. Tetrahedron Lett. 1999, 40, 5327. doi:10.1016/S0040-4039(99)00968-5
Related Reactions
- Merrifield Solid-Phase Peptide Synthesis
- Ugi Reaction for Alternating Peptides
- Mukaiyama Redox Condensation
- Mukaiyama Condensation Reagent
- Yamaguchi Macrolactonization
- Kita Esterification
Related Books
[amazonjs asin=”0080966306″ locale=”JP” title=”Organic Syntheses Based on Name Reactions, Third Edition: a practical guide to 750 transformations”]
External Links
- peptide (Wikipedia)
- Peptide Synthesis (Wikipedia)
- Dicyclohexylcarbodiimide (Wikipedia)
- Carbodiimide (Wikipedia)
- PyBOP (Wikipedia)
- Hydroxybenzotriazole (Wikipedia)
- Condensation Reaction (Wikipedia)
- Steglich Esterification (organic-chemistry.org)
- Japanese version of this article.