- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
–p-Methoxybenzyl (PMB or MPM) group can be protected or deprotected under the same conditions as benzyl group. It can also be deprotected under mildly oxidizing conditions using DDQ (dichlorodicyanobenzoquinone) or strongly acidic conditions.
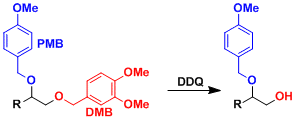
-There are two dimethoxybenzyl (DMB or DMPM) groups (2,4-dimethoxy and 3,4-dimethoxy), both of which can be deprotected under milder conditions than PMB.
-PMB trichloroacetimidate (PMB-O(C=NH)CCl3) provides a way to protect base sensitive compounds under acidic conditions.
-
General References
・Marco, J. L.; Hueso-Rodriguez, J. A. Tetrahedron Lett. 1988, 29, 2459.
・Horita, K.; Abe, R.; Yonemitsu, O. Tetrahedron Lett. 1988, 29, 4139.
・Horita, K.; Yoshioka, T.; Tanaka, T.; Oikawa, Y. Yonemitsu, O. Tetrahedron 1986, 42, 3021.
-
Reaction Mechanism
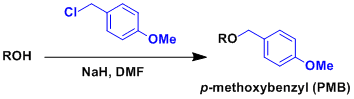
1. Protection
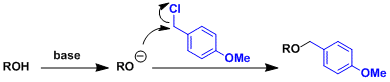
Follows the mechanism of Williamson ether synthesis.
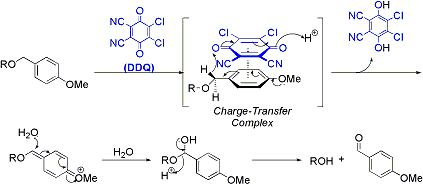
2. Deprotection
Under DDQ conditions, the oxidation of PMB through the charge transfer complex is followed by hydrolysis, releasing the deprotected compound and p-methoxybenzaldehyde.
-
Examples
By reducing a benzylidene (or p-methoxybenzylidene) acetal with DIBAL, a mono-protected diol can be obtained in which the more sterically hindered alcohol is protected.
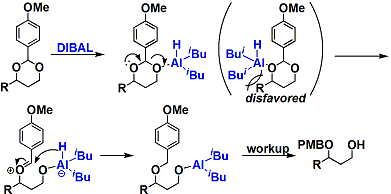
The migration of PMB (or Bn): Using the reductive ring opening reaction of benzylidene acetal described above, one can transfer PMB (or Bn) to the more congested part of the molecule.
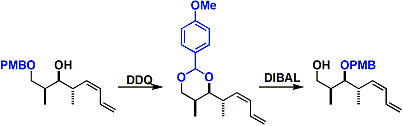
The protecting group generally becomes more labile with increasing number of methoxy substituents on the benzene ring. DMB can be deprotected selectively in the presence of PMB.[1]
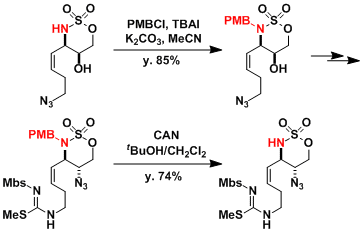
The synthesis of (+)-saxtoxin[2]: Here, the electron-donating property of PMB is used to reduce the electrophilicity of the neighboring sulfonamide, stabilizing the molecule during subsequent transformations.
-
Experimetal Procedure
-
Experimental Tips
-
References
[1] Tetrahedron 1986, 42, 3021.
[2] (a) Fleming, J. J.; Du Bois, J. J. Am. Chem. Soc. 2006, 128, 3926.DOI: 10.1021/ja0608545 (b) Fleming, J. J.; McReynolds, M. D.; Du Bois, J. J. Am. Chem. Soc. 2007, 129, 9964. DOI: 10.1021/ja071501o
-
Related Books
[amazonjs asin=”0471697540″ locale=”US” title=”Greene’s Protective Groups in Organic Synthesis”][amazonjs asin=”0865779937″ locale=”US” title=”Protecting Groups (Foundations of Organic Chemistry)”][amazonjs asin=”0198502753″ locale=”JP” title=”Protecting Group Chemistry (Oxford Chemistry Primers)”]