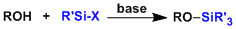- Reagent Availability
- Experimental Ease
- Generality
- Criteria #4
- Criteria #5
-
General Characteristics
-Serving as effective protective groups for alcohols, silyl ethers are used extensively in laboratory scale synthesis.
-At least two of the three R’ groups are the same group, because if all three were different the silicon atom would be stereogenic and could give rise to diastereomers, which would complicate handling of the compound.
-TBS, TIPS, TBDPS groups are bulky enough to selectively protect primary alcohols in the presence of secondary and tertiary alcohols.
-
Reaction Mechanism
1. Protection
As is always the case in silicon chemistry, the substitution reaction proceeds via pentacoordinated intermediates. The elimination of the most electronegative leaving group takes place after it occupies the apical position by pseudorotation.
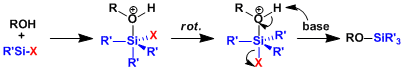
2. Deprotection
Akin to protection, the deprotection proceeds through pentacoodinated intermediates. This is no different under acidic conditions. The reaction mechanism is unlike the SN1 path in carbon chemistry as silyl cations are unstable. The driving force of fluoride-based deprotection is the formation of Si-F bond, which is about 30 kcal/mol stronger than Si-O bond.
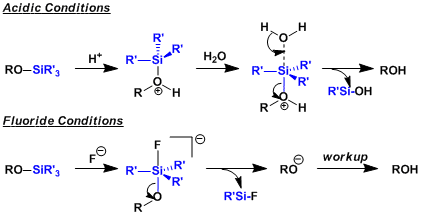
- Examples
Typical examples of protection and deprotection.[1]
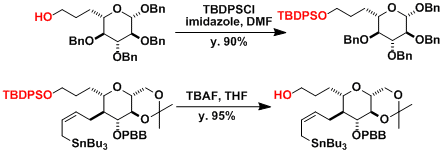
Efficient mono-protection of diols can be done using NaH as the base.[2]
![]()
TMS protection catalyzed by iodine.[3]

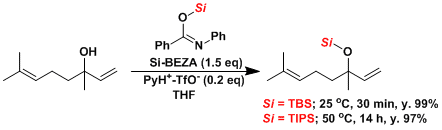
Si-BEZA[4]: Protection of tertiary alcohols under mild conditions.
Silyl ether synthesis catalyzed by tris(pentafluorophenyl)borane[5]: This method is highly functional group tolerant and can protect even sterically hindered alcohols quickly.
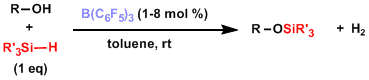
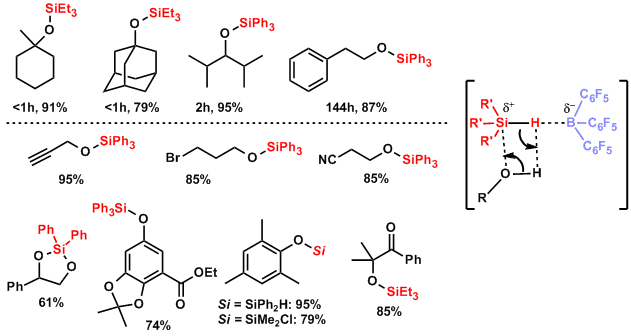
-
Experimental Procedure

<<ADD EXPERIMENTAL LATER>>
–R’3SiCl/imidazole and R’3SiOTf/2,6-lutidine are the common reagent combinations to obtain silyl ethers. The latter is more reactive and suited to the protection of secondary and tertiary alcohols.
-Deprotections based on acidic hydrolysis (AcOH-THF-H2O etc) and fluoride ion (TBAF etc) are used most commonly. The driving force of the latter is the formation of strong Si-F bond.
-
Experimental Tips
-When the reaction is run in DMF, a nice way to work it up is to dilute it with large amount of water at the quench step and extract the product in a hexanes(or petroleum ether)/ethyl acetate mixed solvent. This makes extraction easier as DMF tends to stay in the aqueous layer.
-Common silyl protecting groups are listed below. TBS is generally used as a first choice, but others are also used frequently. TMS is so labile that it is rarely used other than for the protection of sterically hindered alcohols or as temporary protections.

-The relative stability under acidic conditions is TMS<TES<TBS<TIPS<TBDPS, while that against fluoride is TMS<TES<TIPS<TBS<TBDPS.
-TBAF deprotection generates strongly basic ammonium alkoxides that are incompatible with base sensitive compounds. The addition of acetic acid as a buffer or testing milder reaction conditions (HF-pyridine or 3HF-Et3N etc) is needed.
-
References
[1] Oguri, H.; Hishiyama, S.; Oishi, T.;Hirama, M. Synlett 1995, 1252. DOI: 10.1055/s-1995-5259
[2] McDougal, P. G.; Rico, J. G.; Oh, Y.; Condon, B. D. J. Org. Chem. 1986, 51, 3388. DOI: 10.1021/jo00367a033
[3] Karimi, B.; Golshani, B. J. Org. Chem. 2000, 65, 7228. DOI: 10.1021/jo005519s
[4] Misaki, T.; Kurihara, M.; Tanabe, Y.; Chem. Commun., 2001, 2478. doi:10.1039/b107447b
[5] Blackwell, J. M.; Foster, K. L.; Beck, V. H.; Piers, W. E. J. Org. Chem. 1999, 64, 4887. doi:10.1021/jo9903003
[6] Panek, J. S. et al. J. Org. Chem. 2009, 74, 1897.
-
Related Books
[amazonjs asin=”B00B9RYC0W” locale=”US” title=”Greene’s Protective Groups in Organic Synthesis”]