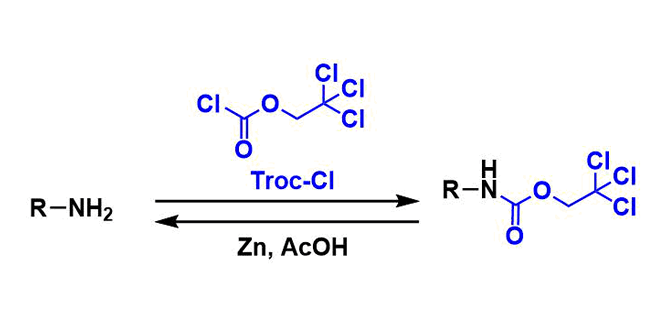
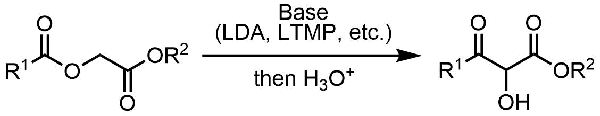General Characteristics 2,2,2-Trichloroethoxycarbonyl (Troc) group is a commonly used carbamate protecting group for amines. It is also used to protect alcohols and phenols. Troc group is stable ...
Author Archive
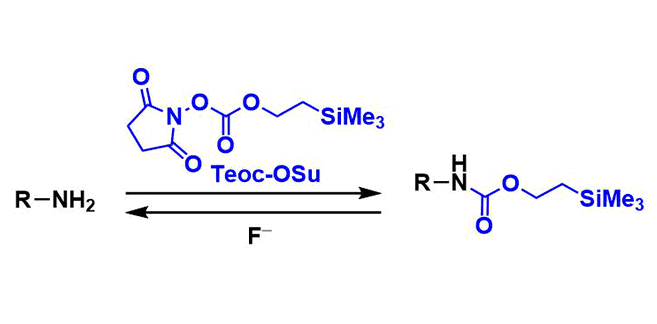
Teoc Protecting Group
General Characteristics 2-(Trimethylsilyl)ethoxycarbonyl (Teoc) group is one of commonly used carbamate protecting groups for amines. It is stable towards hydrolysis and other nucleophilic attacks, ...
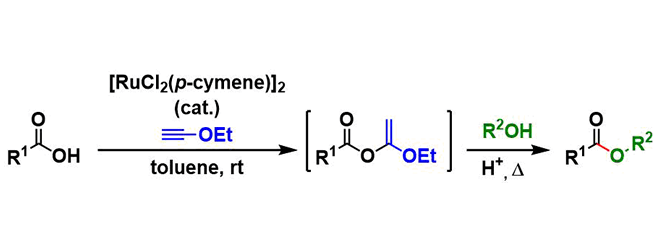
Kita Esterification
General Characteristics Under the conditions developed by Kita, esters are synthesized from the corresponding alcohols and carboxylic acids using a ruthenium catalyst and ethynyl ethyl ether as a ...
Chan Rearrangement
General Characteristics The treatment of α-acyloxyesters with a strong base triggers rearrangement that leads to the formation of α-hydroxy-β-ketoesters. General References S. D. Lee, T. H. Chan, K. ...
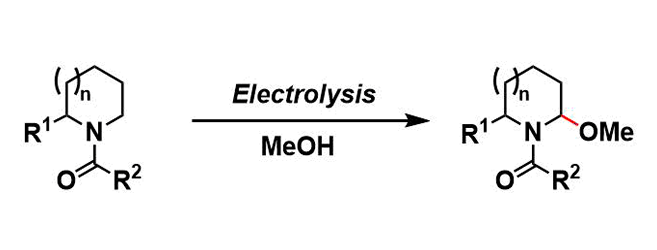
Shono Oxidation
General Characteristics In the Shono oxidation, amides or carbamates in alcohol solvents are electrochemically oxidized to give N,O-acetals. This electrochemical reaction provides a useful way to ...
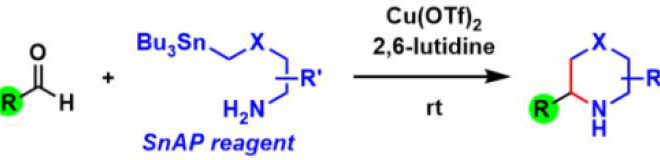
SnAP Reagent
General Characteristics SnAP (Sn(tin) Amine Protocol) reagents provide an easy access to a variety of saturated heterocycles and amines containing a spiro skeleton. The simple two-step procedure ...
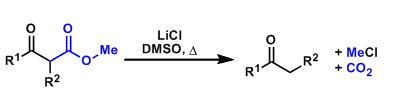
Krapcho Decarboxylation
General Characteristics β-Keto methyl esters undergo decarboxylation under near neutral conditions upon heating in the presence of lithium chloride in DMSO. This reaction is particularly useful when ...
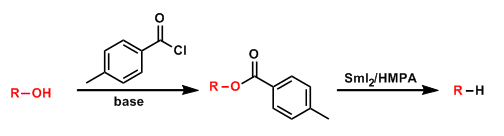
Marko-Lam Deoxygenation
General Characteristics The deoxygenation of alcohols via p-toluic acid esters under single-electron reduction conditions is known as the Marko-Lam deoxygenation. It has advantages over the ...
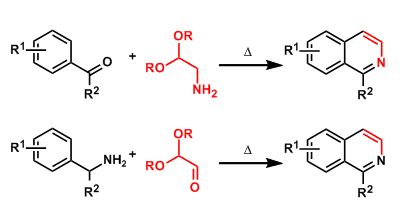
Pomeranz-Fritsch Isoquinoline Synthesis
General Characteristics The condensation of benzaldehydes or arylketones with aminoacetaldehyde acetals gives isoquinolines. Alternatively, the condensation of benzylamines with glyoxal hemiacetals ...
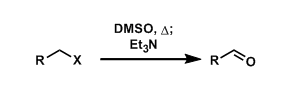
Kornblum Oxidation
General Characteristics Alkyl halides can be converted into the corresponding aldehydes by thermally driven displacement by DMSO followed by treatment with bases. General References ・Kornblum, N.; ...