- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
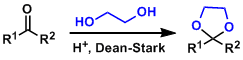
Carbonyl groups are generally protected as acetals under acidic conditions. Acetals are stable under reductive, basic, nucleophilic, and oxidizing (nonacidic) conditions.
-
General References
・Daignault, R. A.; Eliel, E. L. Org. Synth. 1973, 5, 303.
-
Reaction Mechanism
Acetalization is naturally reversible. Use of the alcohols in excess and/or efficient removal of water from the system become important to push the reactions to completion.
The order of reactivity for carbonyl compounds is roughly as follows.
-
Examples
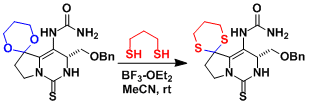
The total synthesis of saxitoxin[1]: Taking advantage of the fact that oxygens are more easily activated by hard Lewis acids or Brønsted acids than sulfurs, the O-acetal was directly converted into the S-acetal.
The Noyori conditions[2]: Acetals or ketals can be synthesized in high yield using the bis-TMS ether reagent and catalytic TMSOTf. This reaction works even at cryogenic temperatures. The disiloxane (TMSOTMS) byproduct is stable and unreactive that the reverse reaction does not occur, allowing for kinetically-controlled protection.
The Otera catalyst[3]: Otera’s distannoxane catalyst mediates acetalization of acid-sensitive compounds. It is proposed that the catalyst forms a nucleophilic tin alkoxide as well as acting as a mild Lewis acid. This reaction does not require any dehydration apparatus.
Ketone-selective acetalization in the presence of aldehyde[4]: The example shown here uses treatment with dimethylsulfide and TMSOTf, followed by the Noyori conditions.
-
Experimental Procedure
The protection of cyclohexanone with ethylene glycol.[5]
-
Experimental Tips
The most popular acetal protecting groups are shown below. The hydrolysis of six-membered ring acetals is faster than that of five-membered ring acetals.
Thioacetals are deprotected mainly by: (1)Methylation then hydrolysis; (2)Oxidation (e.g. by hypervalent iodine) then hydrolysis; (3)Hydrolysis using Hg(II).
-
References
[1] Tanino, H.; Nakata, T.; Kaneko, T.; Kishi, Y. J. Am. Chem. Soc. 1977, 99, 2818. DOI: 10.1021/ja00450a079
[2] Noyori, R.; Murata, S.; Suzuki, M. Tetrahedron 1981, 37, 3899. DOI:10.1016/S0040-4020(01)93263-6
[3] Otera, J.; Dan-oh, N.; Nozaki, H. Tetrahedron 1992, 48, 1449. DOI:10.1016/S0040-4020(01)92233-1
[4] Kim, S.; Kim, Y. G.; Kim, D. Tetrahedron Lett. 1992, 33, 2565. DOI:10.1016/S0040-4039(00)92243-3
[5] Daignault, R. A.; Eliel, E. L. Org. Synth. 1973, 5, 303.
-
Related Books
[amazonjs asin=”0471697540″ locale=”US” title=”Greene’s Protective Groups in Organic Synthesis”]
[amazonjs asin=”1588903761″ locale=”US” title=”Protecting Groups (softcover)”]

![PG_carbonyl_6[1]](https://assets.en.chem-station.com/uploads/2014/04/PG_carbonyl_61.gif)
![PG_carbonyl_8[1]](https://assets.en.chem-station.com/uploads/2014/04/PG_carbonyl_81.gif)
![PG_carbonyl_5[1]](https://assets.en.chem-station.com/uploads/2014/04/PG_carbonyl_51.gif)
![PG_carbonyl_4[1]](https://assets.en.chem-station.com/uploads/2014/04/PG_carbonyl_41.gif)
![PG_carbonyl_9[1]](https://assets.en.chem-station.com/uploads/2014/04/PG_carbonyl_91.gif)
![PG_carbonyl_7[1]](https://assets.en.chem-station.com/uploads/2014/04/PG_carbonyl_71.gif)
![PG_carbonyl_2[1]](https://assets.en.chem-station.com/uploads/2014/04/PG_carbonyl_21.gif)