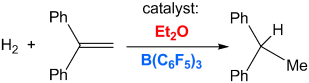Scott, D. J.; Fuchter, M. J.; Ashley, A. E. Angew. Chem. Int. Ed. 53, 2014. 53, 10218.
DOI: 10.1002/anie.201405531
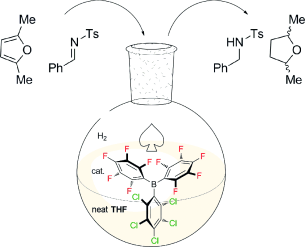
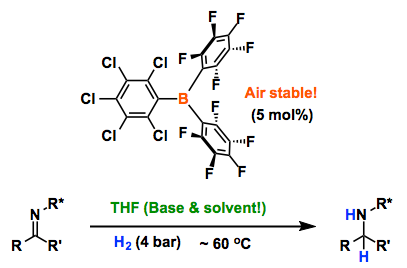
In recent years “frustrated Lewis pairs” (FLPs) have been shown to be effective metal-free catalysts for the hydrogenation of many unsaturated substrates. Even so, limited functional-group tolerance restricts the range of solvents in which FLP-mediated reactions can be performed, with all FLP-mediated hydrogenations reported to date carried out in non-donor hydrocarbon or chlorinated solvents. Herein we report that the bulky Lewis acids B(C6Cl5)x(C6F5)3–x (x = 0–3) are capable of heterolytic H2 activation in the strong-donor solvent THF, in the absence of any additional Lewis base. This allows metal-free catalytic hydrogenations to be performed in donor solvent media under mild conditions; these systems are particularly effective for the hydrogenation of weakly basic substrates, including the first examples of metal-free catalytic hydrogenation of furan heterocycles. The air-stability of the most effective borane, B(C6Cl5)(C6F5)2, makes this a practically simple reaction method.
Since the discovery of the first FLP system by Stephane et al in 2006, [1] finally, air stable version of FLP has appeared! Here, Ashley and co-workers demonstrated catalytic hydrogenation with open air FLP/H2 approach. Although only one experiment was prepared under air in advance, the result showed quantitative catalysis. The key point is that they used more electrophilic but less Lewis acidic B(C6Cl5)(C6F5)2 with respect to B(C6F5)3. They also showed the addition of Lewis base is not required anymore as the reaction solvent (THF) acts as a base.
 The concept to use an ethereal molecule as a Lewis base of FLP has been already demonstrated by Stephane et al [2] but they used a CD2Cl2 solvent at that time. Ashley et al. use THF as not only base but solvent in vast excess. I was wondering why their FLP did not induce polymerization of THF, and noticed something interesting in their Supporting Information. H2 gas in the reaction atmosphere (and of course in reaction solution) seems to affect the behaviour of THF. Thus, the existence of hydrogen prevents THF from oligomerization. To the best of my knowledge, such phenomenon has never been described before. I believe these progress and findings are very important from fundamental point of view.
The concept to use an ethereal molecule as a Lewis base of FLP has been already demonstrated by Stephane et al [2] but they used a CD2Cl2 solvent at that time. Ashley et al. use THF as not only base but solvent in vast excess. I was wondering why their FLP did not induce polymerization of THF, and noticed something interesting in their Supporting Information. H2 gas in the reaction atmosphere (and of course in reaction solution) seems to affect the behaviour of THF. Thus, the existence of hydrogen prevents THF from oligomerization. To the best of my knowledge, such phenomenon has never been described before. I believe these progress and findings are very important from fundamental point of view.
References
[1] “Reversible, Metal-Free Hydrogen Activation”
Welch, G. C.; Juan, R. R. S.; Masuda, J. D.; Stephan, D. W. Science 2006, 314, 1124 – 1126. DOI: 10.1126/science.1134230
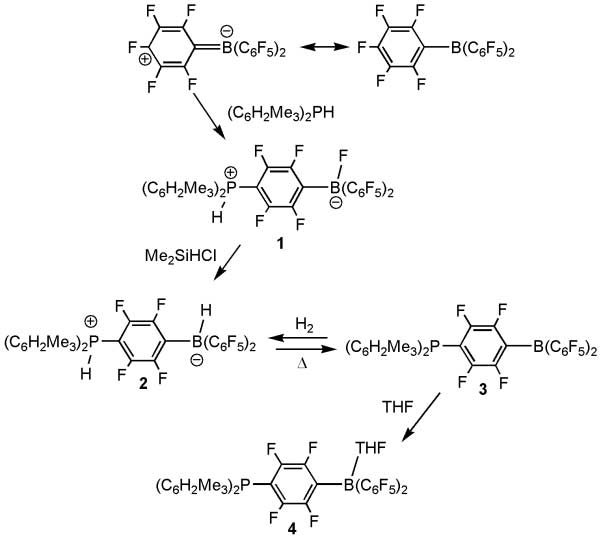
Although reversible covalent activation of molecular hydrogen (H2) is a common reaction at transition metal centers, it has proven elusive in compounds of the lighter elements. We report that the compound (C6H2Me3)2PH(C6F4)BH(C6F5)2 (Me, methyl), which we derived through an unusual reaction involving dimesitylphosphine substitution at a para carbon of tris(pentafluorophenyl) borane, cleanly loses H2 at temperatures above 100°C. Preliminary kinetic studies reveal this process to be first order. Remarkably, the dehydrogenated product (C6H2Me3)2P(C6F4)B(C6F5)2 is stable and reacts with 1 atmosphere of H2 at 25°C to reform the starting complex. Deuteration studies were also carried out to probe the mechanism.
[2] “Combinations of Ethers and B(C6F5)3 Function as Hydrogenation Catalysts”
Hounjet, L. J.; Bannwarth, C.; Garon, C. N.; Caputo, C. B.; Grimme, S.; Stephan, D. W. Angew. Chem. Int. Ed. 52, 2013, 7492 – 7495. DOI: 10.1002/anie.201303166
Labile adducts of dialkyl ethers with the electrophilic borane B(C6F5)3 are shown to scramble HD to H2 and D2 and catalyze the hydrogenation of 1,1-diphenylethylene.
Related Links