A, Fermi.; G, Bergamini.; M, Roy.; M, Gingras.; P, Ceroni.; J. Am. Chem. Soc., 2014, 136 , 6395–6400.
DOI: 10.1021/ja501458s
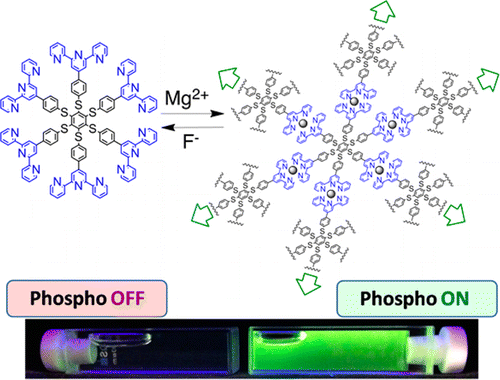
A hexathiobenzene molecule carrying six terpyridine (tpy) units at the periphery has been designed to couple the aggregation induced phosphorescence, displayed by the core in the solid state, to the metal binding properties of the tpy units. Upon Mg2+ complexation in THF solution, phosphorescence of the hexathiobenzene core is turned on. Metal ion coordination yields the formation of a supramolecular polymer which hinders intramolecular rotations and motions of the core chromophore, thus favoring radiative deactivation of the luminescent excited state. Upon excitation of the [Mg(tpy)2]2+ units of the polymeric structure, sensitization of the core phosphorescence takes place with >90% efficiency. The light-harvesting polymeric antenna can be disassembled upon fluoride ion addition, thereby switching off luminescence and offering a new tool for fluoride ion sensing. This unique system can, thus, serve as cation or anion sensor.
Introduction
The phenomenon that molecule begin to emit fluorescence upon aggregation is called aggregation induced emission (AIE).[1] In the paper, Ceroni group in University of Bologna prepared huge complex system where six phenyl-tepryridine[2] are coordinately extended via Mg2+ and evaluated its phosphorescent AIE behavior . 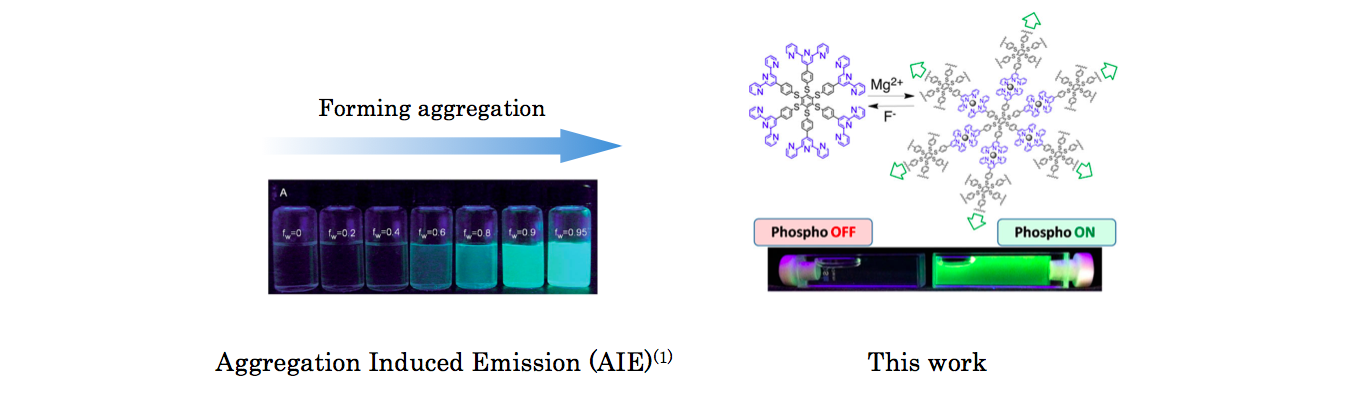

Molecular design
To three dimentionally branched core hexathiobenzene, metal coordinatable terpyridine was covalently attatched. This rational design makes sure that the addition of appropriate metal induces the growth in molecular size and hence aggregation. 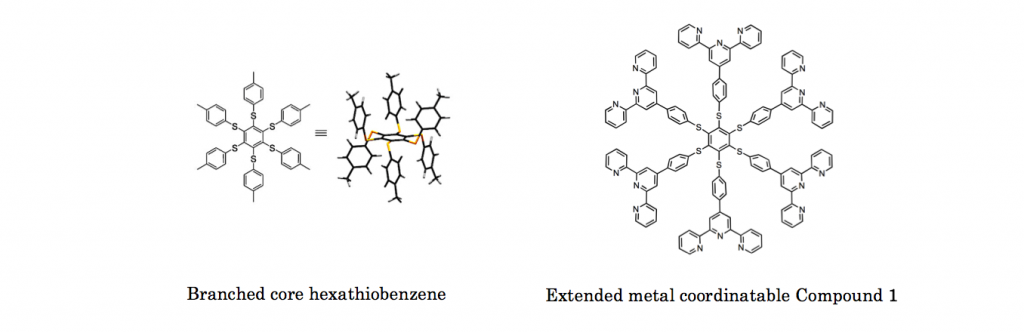

Turn-on phosphorescent AIE
As illustrated below, alternative addition of Mg+2 and F– clearly induced the change in color. Absorption and phosphorescence analysis also showed distinctive changes before and after addition of Mg+2 ion. To the phosphorescence On state, the size distribution experiment was carried out to reveal that the this emission change behavior is classified to AIE. 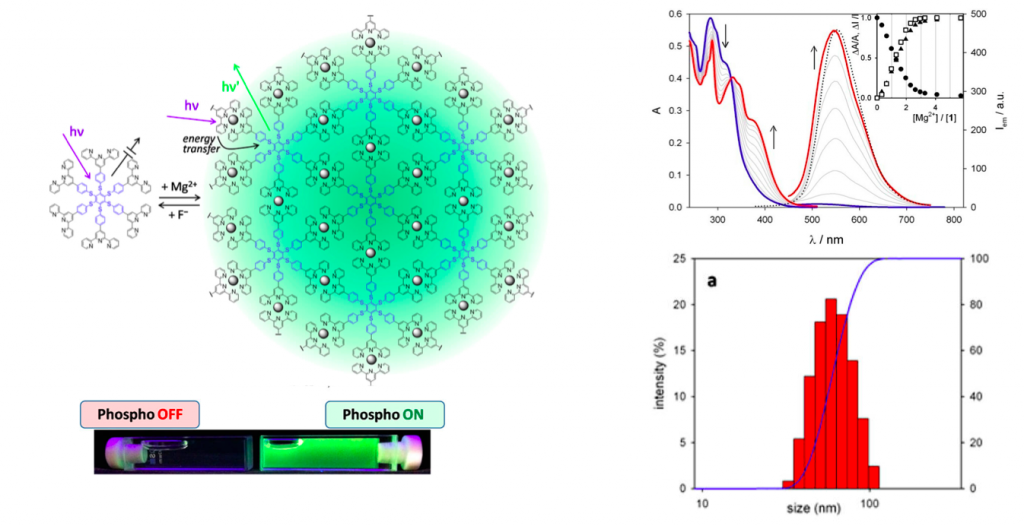

Reference
[1]”Aggregation-induced emission”
Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2011, 40, 5361. DOI: 10.1039/C1CS15113D
[2]”A persulfurated benzene molecule exhibits outstanding phosphorescence in rigid environments: from computational study to organic nanocrystals and OLED applications”
Bergamini, G.; Fermi, A.; Botta, C.; Giovanella, U.; Di Motta, S.; Negri, F.; Peresutti, R.; Gingras, M.; Ceroni, P. J. Mater. Chem. C. 2013, 1, 2717. DOI: 10.1039/C3TC00878A