Those reactions that just won’t proceed…Can’t I brute force this to go?
If you’re pondering the above question, then there may be an interesting solution for you. On this post I’d like to introduce a new microwave reactor that can rapidly enforce extreme conditions to complete a reaction in a controllable manner. Microwave reactors have already become fairly commonplace in the laboratory, and our lab owns one from Biotage (Initiator). While we’ve tried several reactors from other companies, I’ve felt that the one we currently use is among the best with regards to user friendliness and reliability. Anton Paar’s Monowave300 may legitimately challenge Biotage’s supremacy.
-
A Brief Personal Account
Before going further, I’d like to digress a bit and share my personal experience. Microwave reactors existed back in the day when I was a student, but the feeling at the time can be at best described as “questionable” with regard to organic synthesis. Common use of microwaves at the time was instead to, for example, dry molecular sieves using household microwave heaters [which I’m sure many others can relate to].
Moving forward, our lab eventually did decide to get a feel for this instrument , and we purchased one from CEM (Discover, shown below). Just as with any other new toy, I remember running a slew of reactions with the new gizmo. Unfortunately, several nuisances were quickly noticed, which left a bad impression at the time [To be fair, microwave reactors at the time were still relatively new; no offence intended ;)]. These included the following;
- a reactor allowing for only small scale reactions
- poor control over reaction time and temperature
- explosions resulting from overheated chemicals
- frequent malfunctions
Ah, the bitter and sweet(?) memories of the Discover
Moving still forward, my next experience with microwave reactors occurred when I was a post-doc in the US. This was when I got to use Biotage’s [Initiator], and I still remember the reliability and ease at which I was able to use the touch panel to control my reactions. Today we make great use of this instrument in our group, especially since we frequently encounter reactions that proceed very slowly. Particularly appealing is being able to avoid using a reflux condenser under closed conditions.
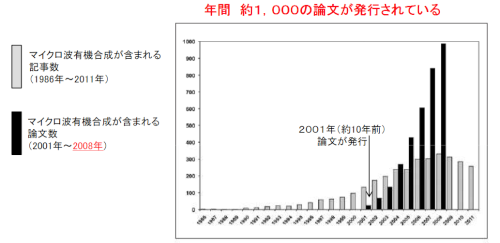
While there’s still debate as to how a reaction inside a reactor is actually accelerated, use of microwave reactors in organic synthesis has steadily increased over the years [Figure below]. In 2008, around 1000 papers involving it’s use were published! (whose figure is sure to be surpassed in 2014).
Number of papers which mention the use of a microwave reactor
-
Anton Paar’s Microwave Reactor [Monowave300]
So finally moving to the main subject of this post, I one day received a call from Anton Paar Japan with a request to test their newly developed microwave reactor. I would have declined under normal circumstances, especially since test trials do consume a measurable amount of time; this case, however, was different in that the instrument was also recommended by a good friend, who claimed that he’d become a regular user after it’s purchase.
So what exactly is Anton Paar Japan anyway? You’re not alone if you thought so, since this was precisely the question that sprung up in my mind.
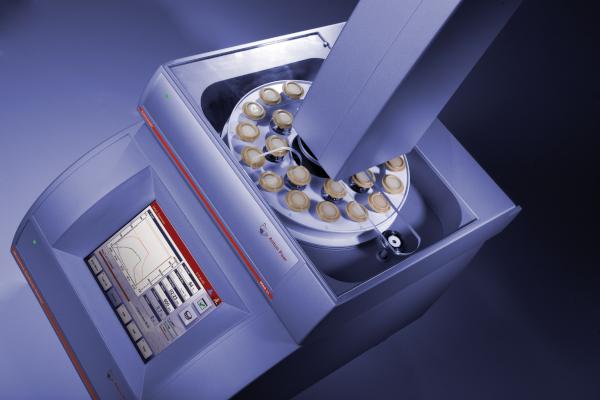
If you look up their website, Anton Paar’s headquarters is located in Austria, and they specialize in high accuracy measurement hardware both for industry and lab work. The Monowave300, which is the instrument we used in the test trial, was developed by a former student of Prof. Oliver Kappe, a well known figure in the field of organic synthesis with microwave radiation. In essence, the Monowave 300 embodies an organic chemist’s full blown desire for rapid synthesis. Just for reference, its appearance is rather stylish, and it is relatively small.
-
Feedback
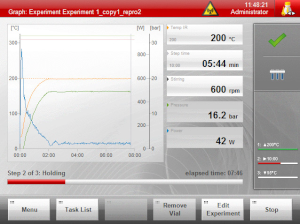
I’ve had several students use this instrument, and they seemed to become quickly comfortable with it, uniformly leaving positive feedback. The main touch screen is very easy to follow, clealy showing temperature, pressure, applied voltage, power, and progress of reaction. It may be described as a modernized version of the Initiator.
Main Control Screen
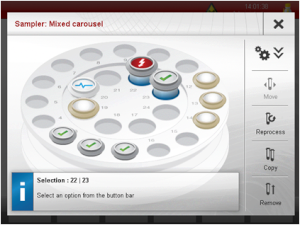
On to the auto-sampler. Most microwave reactors allow for a max reaction scale of around 20mL. Unfortunately, the Monowave300 is no exception. However, use of the auto-sampler and running reactions in parallel allows for a total reaction scale that well surpasses 200~300mL. Setting a reaction container onto the auto-sampling requires simply placing it on top. In addition, the auto-sampler screen shown below, is also very easy to follow.
Auto-sampler control screen
A brief comment on the reaction vessel. The vessel, appearance-wise, is fairly similar to ones by other companies, but is rather robust. For those requiring higher robustness, they even have vessels made from silicon carbide. Just as other instruments, the size of reaction vessels they offer covers a wide range.
Reaction vessel
A prominent feature is the vessel cap. The cap requires no special apparatus to fix in position, as other designs do. Simple use of your hands is more than enough to cap and remove. The cap is also designed to vent in cases of overpressure, avoiding explosions resulting from such cases. Quite appealing, I’m sure, for those who had to send their instrument off to repair after such explosions.
An easily removable cap
Finally, some comment on the strength of microwave radiation. The Monowave300 allows for higher power than other reactors, featuring a max output of 850W (230V). Any solvent may be heated in a similar manner. I speak from experience, as I found that the desired temperature was smoothly and quickly attained after starting. Other features include a built in temperature sensor, and you can even place a camera (optional feature) to monitor your reaction visually.
With all the good things said about the instrument, several disadvantages do exist. So let me summarize the pros and cons.
Pros
User friendly main and auto-sampler control screen
Easily manipulated snap cap
Visualization of reaction through use of optional camera
Meticulous safety design that allows for facile high temperature chemistry (silicon carbide vessel available)
Cons
Need of a 230V power source to attain max output (850W)
Less known relative to major competitors and thus, reliability not fully assessed
Additional space required for an air compressor used for cooling. Leads to noise when cooling.
While several cons do exist, the price of the instrument with the auto-sampler included, is incredibly low. I feel that this instrument could become a major player in organic synthesis if such disadvantages could be overcome (or likewise, if the pros could significantly outweigh the cons). Just for reference, I’ve recently heard that another research lab bought the Monowave300 after its demo-use. Purchase is also under consideration in our group.
With that, I’d like to conclude the introduction of the Monowave300 made by Anton Paar. If you’re found this instrument to be interesting, I highly recommend that you try it out for a week. It could become your BFF. Perhaps mentioning that you’ve seen the ChemStation article could lower its price during negotiations(!?)
Contact Anton Paar GmbH
Anton Paar Strasse 208054 GRAZAUSTRIA
Tel.+43 316 257 0Fax +43 316 257 257
Email: ASC@anton-paar.com
Website about microwave reactors: www.anton-paar.com/corp-en/products/group/microwave-synthesis/
Website about microwave synthesis: www.this-is-synthesis.com