DOI: 10.1021/ja505444d
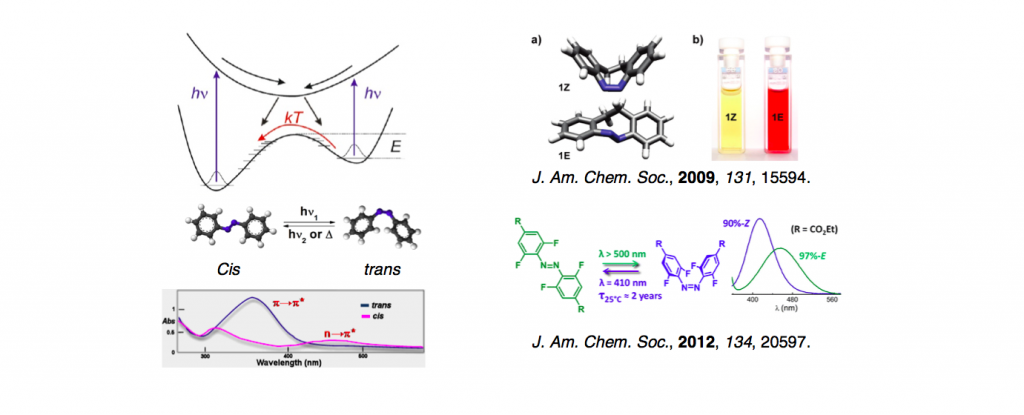
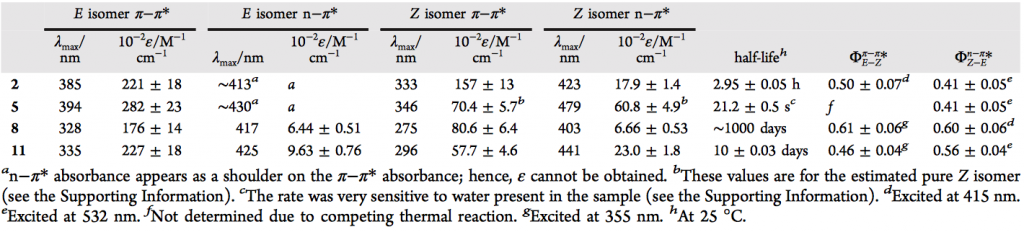
Arylazopyrazoles, a novel class of five-membered azo photoswitches, offer quantitative photo- switching and high thermal stability of the Z isomer (half- lives of 10 and ∼1000 days). The conformation of the Z isomers of these compounds, and also the arylazopyrroles, is highly dependent on the substitution pattern on the heteroarene, allowing a twisted or planar geometry, which in turn has a significant impact on the electronic spectral properties of the compounds. <Introduction> Azobenzene is the most famous and historical photochromic molecule. Because of its two-valley shaped potential surface in grand state: trans-form is more stable than cis-form and single shared excited stated, azobenzenes derivatives in its cis-form converge in initial (more stable) trans-form by thermal effect. Additionally, they often suffer from absorption overlap of both isomers, which make less suitable for memory storage candidate. Although various azobenzenes with improved properties have been report so far,[1] the advent of new derivatives with large separation of both absorption spectrum and highly thermal stability is still in demand.
Introduction
Azobenzene is the most famous and historical photochromic molecule. Because of its two-valley shaped potential surface in grand state: trans-form is more stable than cis-form, azobenzenes derivatives in its cis-form converge in initial (more stable) trans-form by thermal effect. Additionally, they often suffer from absorption overlap of both isomers, which make less suitable for memory storage candidate. Although various azobenzenes with improved properties have been report so far,[1] the advent of new derivatives with large separation of both absorption spectrum and highly thermal stability is still in demand.
Photochemistry of four derivatives
Theoretical calculation
References
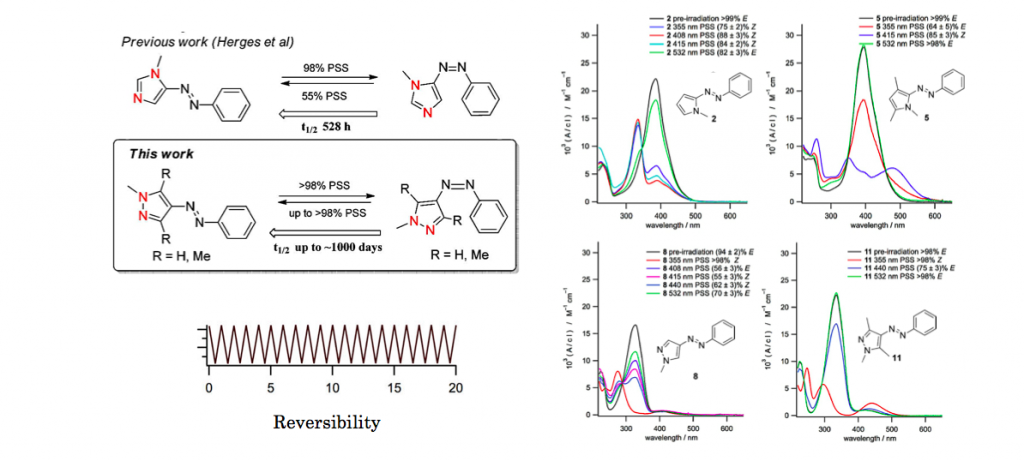
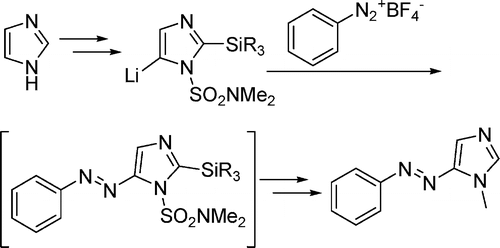
In contrast to azobenzenes, heterocyclic azo compounds are less well investigated. Phenylazoimidazoles would be versatile as photodissociable ligands (PDLs) because imidazole is an important donor in coordination chemistry. Here, we present the synthesis of 4- and 5-phenylazoimidazoles via a novel azo-coupling method. 1,2-Protected imidazole is lithiated in the 5-position and coupled with benzenediazonium tetrafluoroborate. Several new phenylazoimidazoles were prepared. They exhibit an excellent switching behavior. Upon irradiation of the trans isomers with UV light, >95% of the cis forms are obtained. Upon heating, a complete transformation back to the trans configuration was achieved. Back switching with visible light, however, is incomplete.
[2]”Photoisomerization in different classes of azobenzene”
Bandara, H. M. D.; Burdette, S. C. Chem. Soc. Rev. 2012, 41, 1809. DOI: 10.1039/C1CS15179G
Azobenzene undergoes trans → cisisomerization when irradiated with light tuned to an appropriate wavelength. The reverse cis →transisomerization can be driven by light or occurs thermally in the dark. Azobenzene’s photochromatic properties make it an ideal component of numerous molecular devices and functional materials. Despite the abundance of application-driven research, azobenzene photochemistry and the isomerization mechanism remain topics of investigation. Additional substituents on the azobenzene ring system change the spectroscopic properties and isomerization mechanism. This critical review details the studies completed to date on the 3 main classes of azobenzene derivatives. Understanding the differences in photochemistry, which originate from substitution, is imperative in exploiting azobenzene in the desired applications.