W, Cho.; H, Lee.; G, Choi.;S, Choi.; M, Oh. J. Am. Chem. Soc., 2014, 136, 12201.
DOI: 10.1021/ja504204d
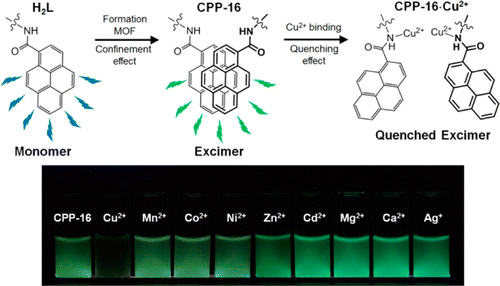
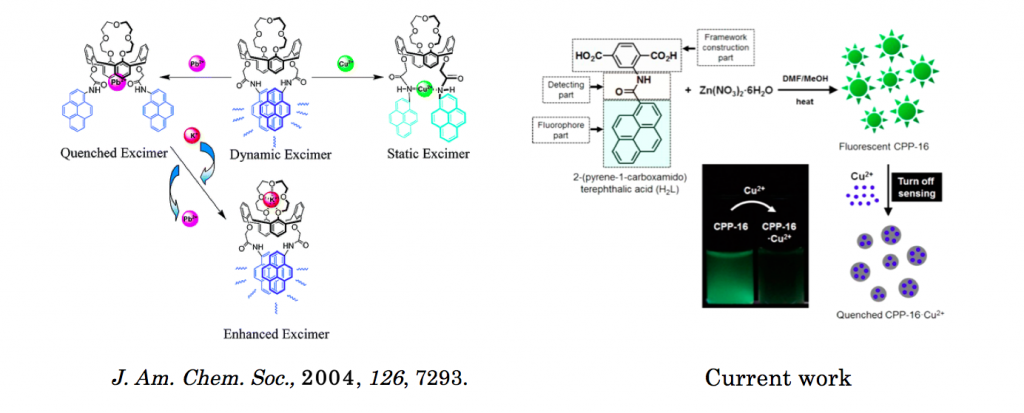
Microsized chemosensor particle (CPP-16, CPP means coordination polymer particle), which is made from a metal−organic framework (MOF), is synthesized using pyrene-functionalized organic building block. This building block contains three important parts, a framework construction part, a Cu2+ detection part, and a fluorophore part. PXRD studies have revealed that CPP-16 has a 3D cubic structure of MOF-5. During both MOF formation and sensing event, fluorophores within CPP-16 undergo dual changes in conformation and optical properties. After MOF construction, pyrene moieties experience an unusual complete conversion from monomer to excimer form. This conversion takes place due to a confinement effect induced by space limitations within the MOF structure. The selective sensing ability of CPP-16 on Cu2+ over many other metal ions is verified by emission spectra and is also visually identified by fluorescence microscopy images. Specific interaction of Cu2+ with binding sites within CPP- 16 causes a second conformational change of the fluorophores, where they change from stacked excimer (CPP-16) to quenched excimer states (CPP-16·Cu2+).
Introduction
Pyrene excimer functionalized fluorescent chemosonser that can detect the presence of metals has been studied extensively.[1] Among other metal sensors, the creation of solid state metal sensor is highly anticipated because as well as detecting metal they are also capable of capturing them and reused.[2] Moonhyun group at Yonsei University synthesized solid-state pyrene-functionalized MOF which can selectively sensing Cu2+.
MOF design
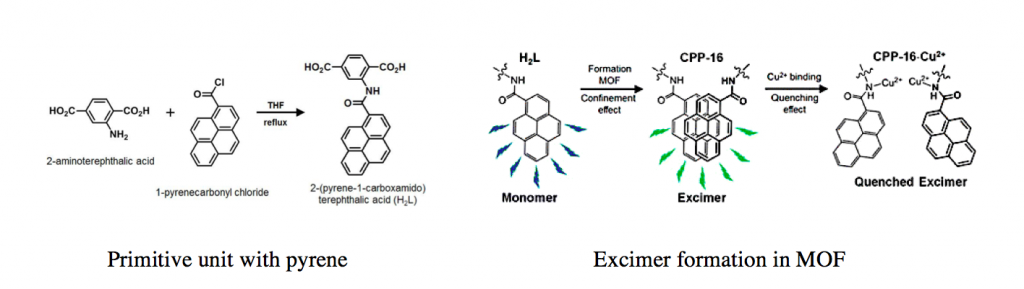
MOF primitive unit was synthesized by simply connecting pyrene and terephthalic acid through peptide bond. Here nitrogen plays vital role in detecting Cu2+ hence deciding whether pyrene form excimer or not.
Structural analysis and metal ion detection
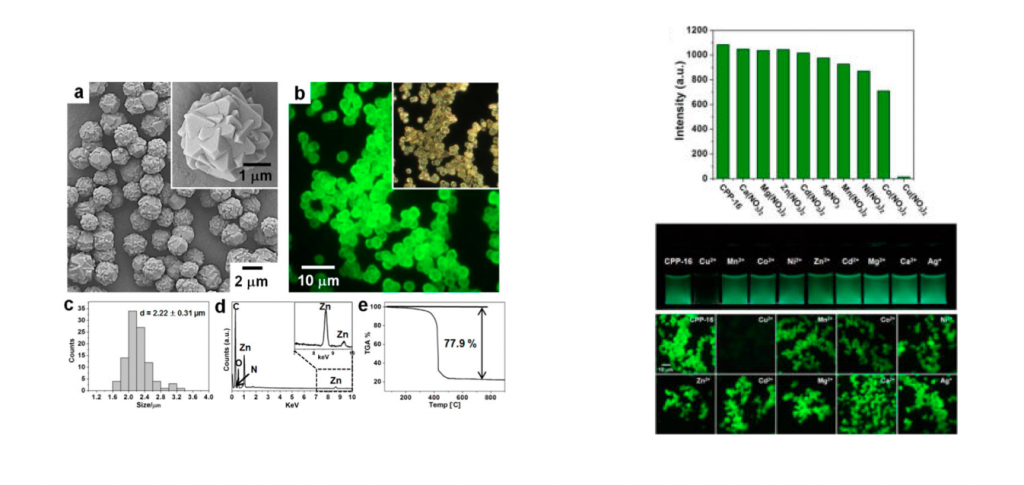
The pyrene coded MOF was subjected to analysis like field-emission scanning electron microscopy (FE-SEM), optical microscopy (OM), fluorescence microscopy (FM), and so on. As expected the data showed that the pylene coded MOF is in particle state and emit green fluorescence. In the last various metal was dropped to the prepared MOF to see its fluorescence behavior. As shown in the figure below, only addition of Cu2+ ion broke the excimers in MOF and dramatically bleached the green fluorescence.
Reference
[1]” An Excimer-Based, Binuclear, On−Off Switchable Calix[4]crown Chemosensor”
Kim, S. K.; Lee, S. H.; Lee, J. Y.; Lee, J. Y.; Bartsch, R. A.; Kim, J. S. J. Am. Chem. Soc.2004, 126, 16499. DOI: 10.1021/ja045689c
[2]” Zeolitic imidazolate framework-8 as a luminescent material for the sensing of metal ions and small molecules”
Liu, S.; Xiang, Z.; Hu, Z.; Zheng, X.; Cao, D.J. Mater. Chem. 2011, 21, 6649.DOI: 10.1039/C1JM10166H