K, Li.; Y, Xiang.; X, Wang.; J, Li.; R, Hu.; A, Tong.; B, Tang. J. Am. Chem. Soc., 2014, 136, 1643–1649.
DOI: 10.1021/ja411689w
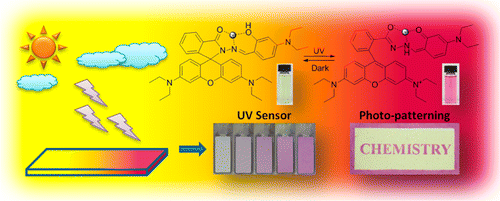
ABSTRACT: Photochromic molecules are widely applied in chemistry, physics, biology, and materials science. Although a few photochromic systems have been developed before, their applications are still limited by complicated synthesis, low fatigue resistance, or incomplete light conversion. Rhodamineis a class of dyes with excellent optical properties including long-wavelength absorption, large absorption coefficient, and high photostability in its ring-open form. It is an ideal chromophore for the development of new photochromic systems. However, known photochromic rhodamine derivatives, such as amides, exhibit only millisecond lifetimes in their colored ring-open forms, making their application very limited and difficult. In this work, rhodamine B salicylaldehyde hydrazone metal complex was found to undergo intramolecular ring-open reactions upon UV irradiation, which led to a distinct color and fluorescence change both in solution and in solid matrix. The complex showed good fatigue resistance for the reversible photochromism and long lifetime for the ring-open state. Interestingly, the thermal bleaching rate was tunable by using different metal ions, temperatures, solvents, and chemical substitutions. It was proposed that UV light promoted isomerization of the rhodamine B derivative from enol-form to keto-form, which induced ring- opening of the rhodamine spirolactam in the complex to generate color. The photochromic system was successfully applied for photoprinting and UV strength measurement in the solid state. As compared to other reported photochromic molecules, the system in this study has its advantages of facile synthesis and tunable thermal bleaching rate, and also provides new insights into the development of photochromic materials based on metal complex and spirolactam-containing dyes.
Introduction
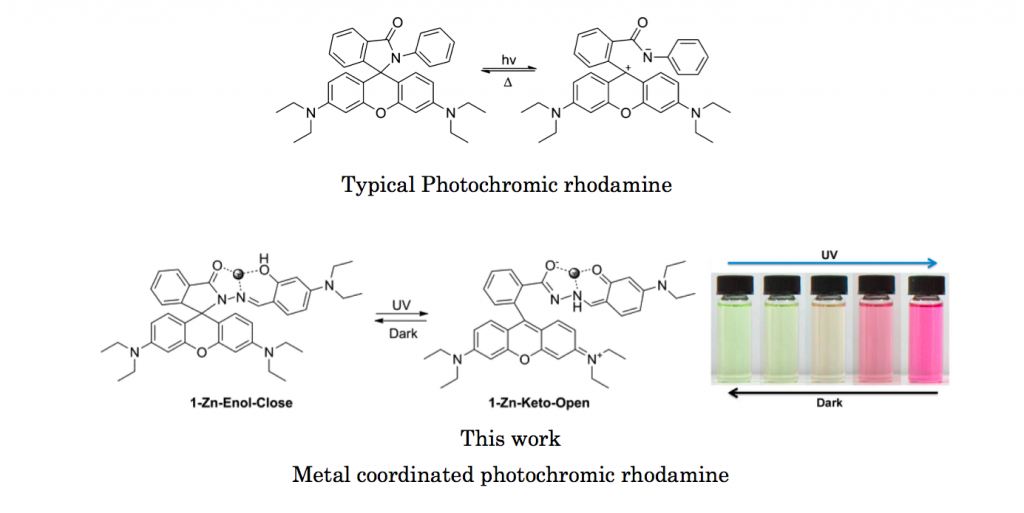
Since its first discovery 1977, photochromism of rhodamine has attracted much attention in many branches chemistry. Various derivatives with wide selection in absorption region have been prepared so far. In this work, Tang group at the Hong Kong University prepared and evaluated new metal coordinatable photochromic rhodamine.
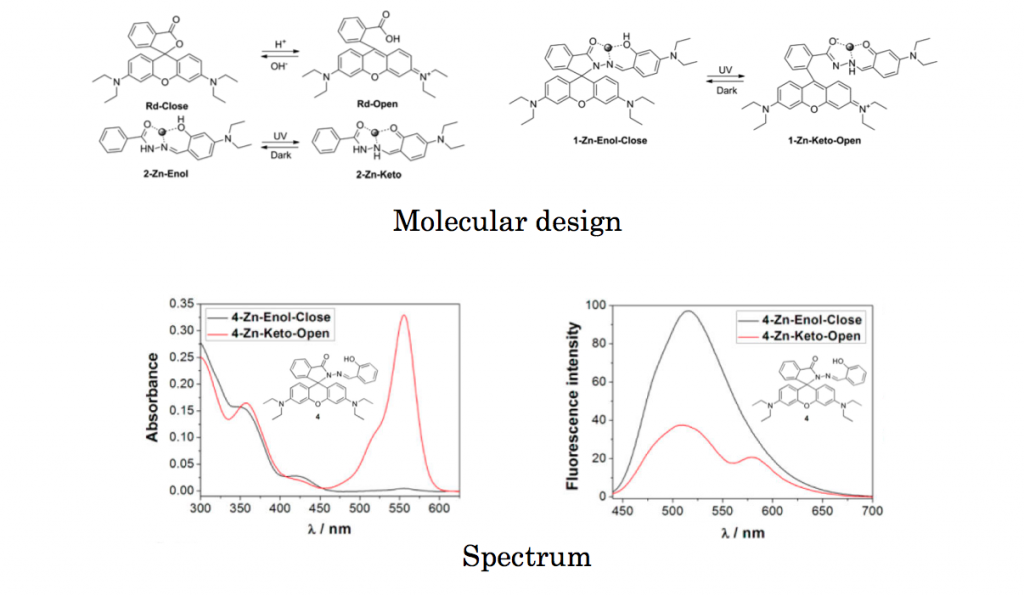
Molecular design and spectrum
Salicylaldehyde Schiff bases[1] which shows photochromic reaction between Enol and Keto forms was embedded into rhodamine. Thus the resulting target compound is composed of two photochromic units. Photoirradiation experiment was carried out to both before and after addition of metal. It was revealed that Schiff bases which is capable of metal coordination is essential to achieve smooth photochromic reaction in this newly developed system.
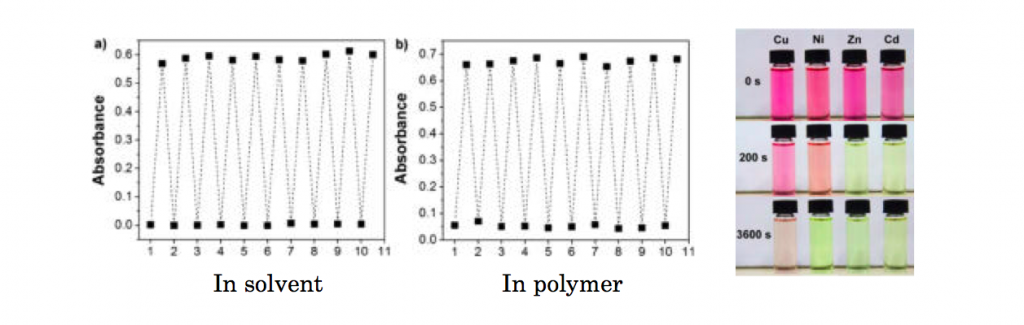
Reversibility and thermal stability
The reversibility between colorless and colored form upon alternate light exposures were examined in both solid and polymer state. The experiment revealed that the system had a excellent switching ability and suitable for practical uses. On top of that, the prepared compound had is coordinatable to various metals and the thermal stability heavily depends on the coordinated metals.
References
[1]” Photochromism and thermochromism of Schiff bases in the solid state: structural aspects”
Hadjoudis, E.; Mavridis, I. M. Chem. Soc. Rev. 2004, 33, 579. DOI: 10.1039/B303644H