S, Kim.;T, Tachikawa.; M, Fujitsuka.; T, Majima. J. Am. Chem. Soc. 2014. 136, 11707.
DOI: 10.1021/ja504279r
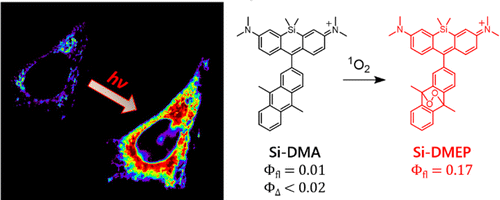
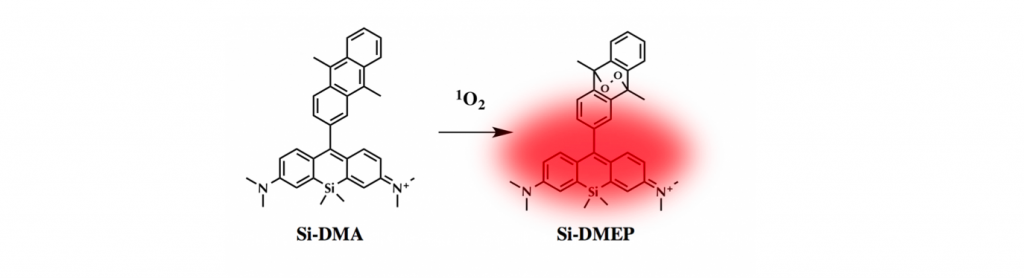
Singlet oxygen (1O2), molecular oxygen in thelowest excited state, has a critical role in the cell-killing mechanismof photodynamic therapy (PDT). Although 1O2 phosphorescencemeasurement has been mainly used to monitor 1O2 formationduring PDT, its intensity is far insufficient to obtain two-dimensional images of intracellular 1O2 with the subcellular spatialresolution using the currently available near-IR detector. Here, wepropose a new far-red fluorescence probe of 1O2, namely, Si-DMA,composed of silicon-containing rhodamine and anthracenemoieties as a chromophore and a 1O2 reactive site, respectively. In the presence of 1O2, fluorescence of Si-DMA increases 17 times due to endoperoxide formation at the anthracene moiety. With the advantage of negligible self-oxidation by photoirradiation (ΦΔ < 0.02) and selective mitochondrial localization, Si-DMA is particularly suitable for imaging 1O2 during PDT. Among three different intracellular photosensitizers (Sens), Si-DMA could selectively detect the 1O2 that is generated by 5- aminolevulinic acid-derived protoporphyrin IX, colocalized with Si-DMA in mitochondria. On the other hand, mitochondria- targeted KillerRed and lysosomal porphyrins could not induce fluorescence change of Si-DMA. This surprising selectivity of Si- DMA response depending on the Sens localization and photosensitization mechanism is caused by a limited intracellular 1O2 diffusion distance (∼300 nm) and negligible generation of 1O2 by type-I Sens, respectively. For the first time, we successfully visualized 1O2 generated during PDT with a spatial resolution of a single mitochondrial tubule.
Introduction

PDS photodynamic therapy is widely practiced medical treatment. Typical PDS takes advantage of ROS (reactive oxygen species) that are spontaneously generated from organic probe used during the treatment. To be specific, 1O2 is recognized as the main cause of cell death because other ROS like HO• and O2•- are generated via 1O2.[1] Hence, detection and visualization of 1O2 molecule location within living cell are very important for further its development and understanding. Here, Majima group at Osaka university realized the live-visualization of 1O2 using silicon-containing rhodamine.
Molecular design
In this paper, high photo-stable, mitochondria selective, red emissive and 1O2 selective dye was reported. The new dye was designed as shown below.
(1) Considering its photostability and photo-physical properties, silicon based fluorescein was adopted in core fluorophore.
(2) The often used phenyl group in the head part was replaced with 9,10-dimethylanthracene (DMA) because it react with 1O2 with high selectivity.
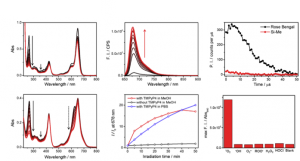
 In vitro characterization
In vitro characterization
As shown below, anthracene’s band in short wavelength region dramatically ceased when 1O2 reagent was added. Simultaneously, increase in fluorescence intensity was also observed, meaning that the oxygen bridgedstructure was formed on anthracene, which hampered 1O2 transfer from the main fluorophore. Absorption spectrum change suggested that the dye formed H-aggregation in PBS buffer. Last date showed that the dye has the highest selectivity to 1O2 among other ROS species.
In vivo experiment
Comparison with Mitotracker green revealed that the Si-DMA had specific localization in mitochondria. Next, Si-DMA was stained together with PpIX and TMPyP4 and KillerRed for its further characterization. After co-stained followed by appropriate wavelengths photoirradiation, only PpIX stained with Si-DMA showed dramatic change in fluorescence intensity. These results revealed that Si-DMA can selectively detect 1O2 in mitochondria.
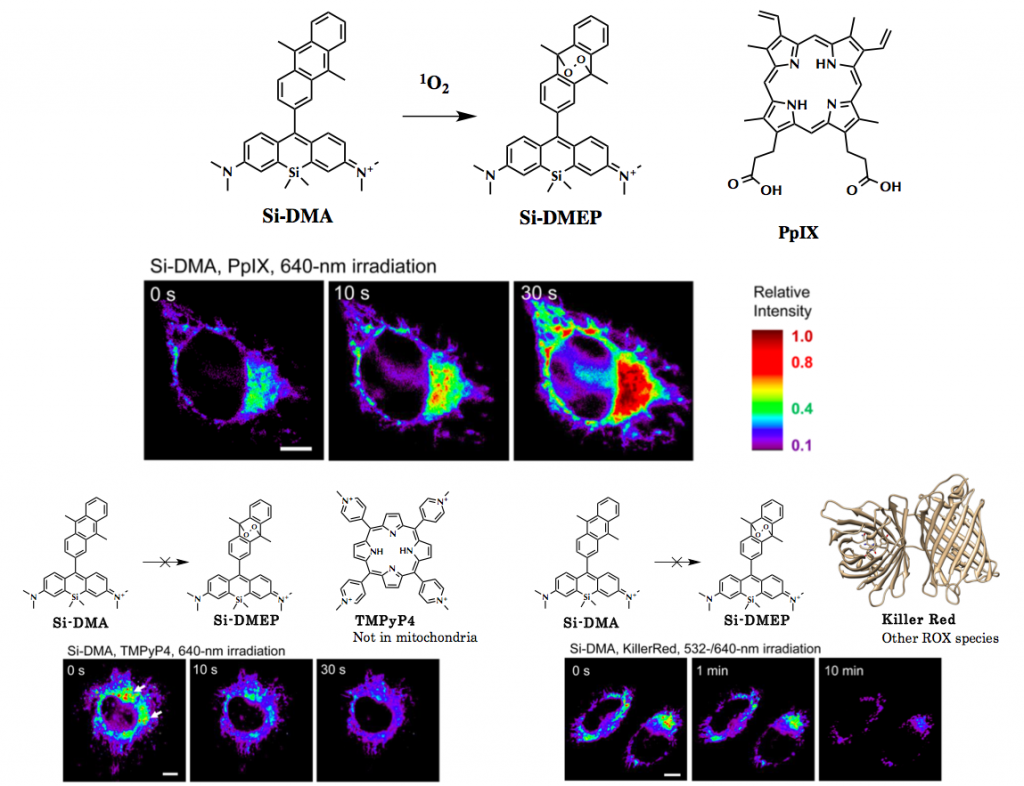 PpIX is pervasively used PDS that which localizes in mitochondria. TMPyP4 is also known to generate1O2but it selectively localize in lysosome not mitochondria. KillerRed is PDS protein that unlike other dyes generates superoxide anion rather than 1O2.[2]
PpIX is pervasively used PDS that which localizes in mitochondria. TMPyP4 is also known to generate1O2but it selectively localize in lysosome not mitochondria. KillerRed is PDS protein that unlike other dyes generates superoxide anion rather than 1O2.[2]
References
[1]”Singlet oxygen: there is indeed something new under the sun”
Ogilby, P. R. Chem. Soc. Rev. 2010, 39, 3181. DOI: 10.1039/c4pp00090k
[2]”Reactive oxygen species in photochemistry of the red fluorescent protein ‘‘Killer Red’’
Vegh, R. B.; Solntsev, K. M.; Kuimova, M. K.; Cho, S.; Liang, Y.; Loo, B. L. W.; Tolbert, L. M.; Bommarius, A. S. Chem. Commun. 2011, 47, 4887.
DOI: 10.1039/c0cc05713d