- Generality
- Reagent Availabiltiy
- Experimental User Friendliness
- Criteria #4
- Criteria #5
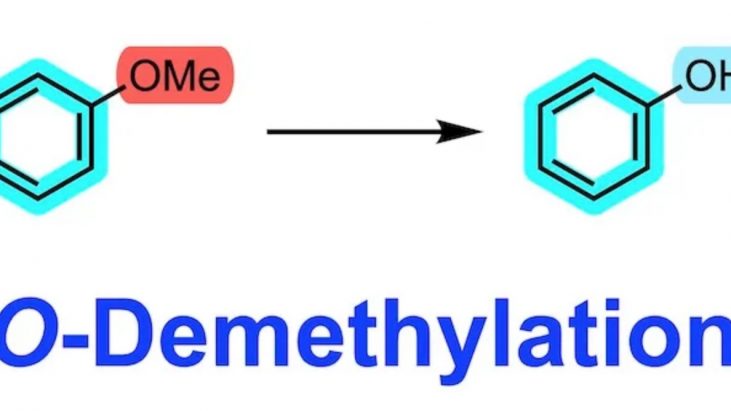
Conversion of a methoxy group to a hydroxy group is sometimes a rather difficult task. The methyl group (alkyl group) is too stable to call it a protecting group, but synthesis from inexpensive commercial reagents may be forced to perform a demethylation reaction.
Considering the harsh conditions for demethylation, it would be better to perform the demethylation reaction of the methoxy group as early in the synthesis as possible and quickly replace it with another protecting group.
Option 01. BBr3
The first choice would be boron tribromide BBr3. BBr3 is a very strong Lewis acid, and an electron pair of the O atom attacks the empty orbital of the boron atom to form a complex, giving rise to a formal O cation. The released bromo-anion then attacks the methyl group to form bromomethane and alkoxydibromoborane. Alkoxydibromoborane is hydrolyzed to give the corresponding hydroxy compound.
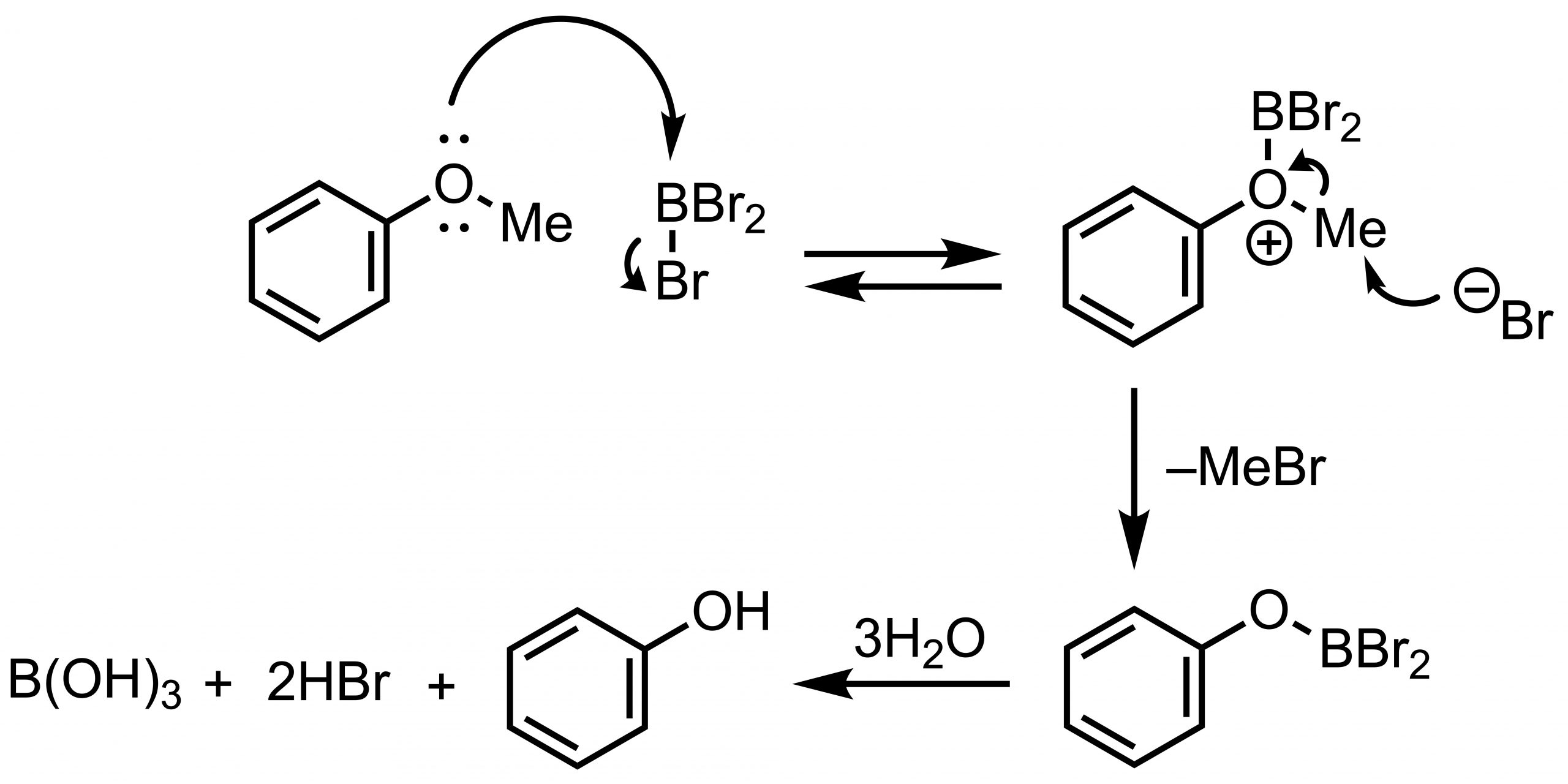
Because of the high reactivity of BBr3, it is common to start the reaction under low-temperature conditions (-78˚C – 0˚C) and gradually increase the temperature while checking the progress. Since BBr3 reacts violently with water, extreme care must be taken during quenching. In recent years, dichloromethane solutions of BBr3 (ca. 1 M) are commercially available from various companies.
Even dichloromethane solutions emit a lot of smoke. Also, the septum becomes reddish-black and burnt. Another problem is that the reagent in solution form is relatively expensive.
Option 02. AlCl3
Like boron tribromide, AlCl3 is a strong Lewis acid, but its reactivity is lower. AlCl3 is often used in Friedel-Crafts acylation reaction. In dichloromethane, O-demethylation may proceed simply by mixing with the substrate and heating, but various improved methods have also been reported. Reactions in acetonitrile seem to give good results.[1]
Precautions when using AlCl3 include (1) handling it in a draft with a mask because of the hydrogen chloride fumes when the bottle is opened, and (2) weighing it in a mortar and grinding it quickly before use because it is deliquescent and its surface is often inactivated by an oxidized film.
Option 03. 47% HBr
47% HBr is a common method of demethylation using Brønsted acids. The reaction mechanism is simple: the oxygen atom is protonated, and the bromide anion attacks the methyl group to produce the O-demethylated product and bromomethane.
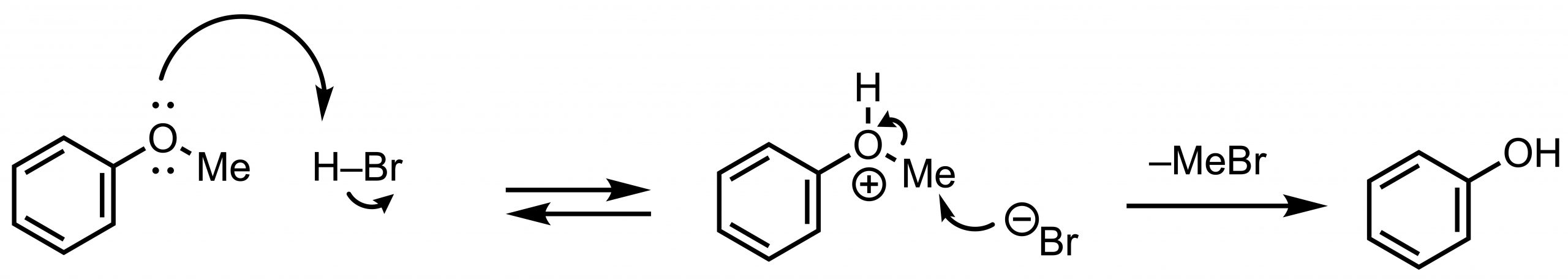
A 47% HBr solution is added directly to the substrate and heated to about 130˚C. Acetic acid can be added as a solvent if the substrate does not dissolve well. Variations have also been reported on adding lithium bromide LiBr [2] or aliquat 336.[3] Both aqueous solution and acetic acid solution are available from commercial companies.
Option 04. Alkyl thiols
O-Demethylation using alkyl thiols is a useful reaction that does not require the use of strong acids. A classical method is to use the ethane thiol (EtSH) under basic conditions (e.g., NaOH), but the odor peculiar to low thiols is problematic. Chae reported an example using the odorless 1-dodecanethiol CH3(CH2)11SH.[4]
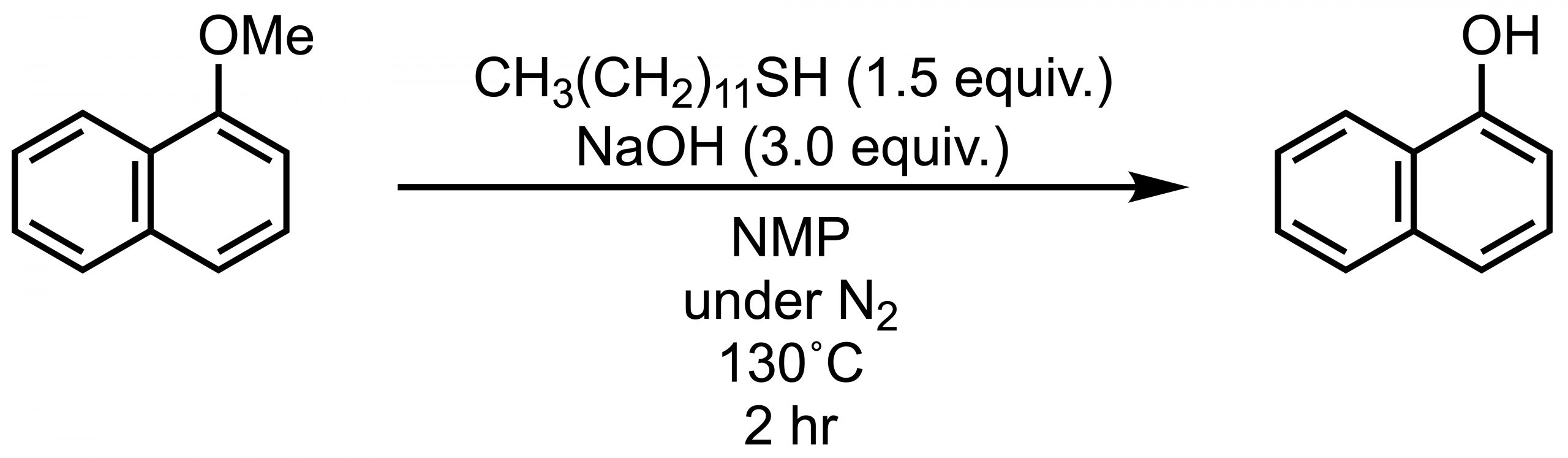
This reaction does not proceed in THF or 1,4-dioxane, even under reflux conditions, but heating at 130˚C in a high-boiling solvent such as NMP or DMSO gives good yields of over 90%. NMP seems better than DMSO because some byproducts are produced in DMSO. Drawbacks are the requirement of a nitrogen atmosphere and incompatibility with heat-sensitive compounds.
References
- Sang, D.; Tu, X.; Tian, J.; He, Z.; and Yao, M., “Anchimerically Assisted Cleavage of Aryl Methyl Ethers by Aluminum Chloride-Sodium Iodide in Acetonitrile”, ChemistrySelect, 2018, 3, 10103-10107, DOI; 10.1002/slct.201802565.
- Li, Z.; Sutandar, E.; Goihl, T.; Zhang, X.; Pan, X., “Cleavage of ethers and demethylation of lignin in acidic concentrated lithium bromide (ACLB) solution”, Green Chem., 2020, 22, 7989-8001, DOI: 10.1039/d0gc02581j.
- Waghmode, S.B.; Mahal, G.; Patil, V.P.; Renalson, K.; Singh, D., “Efficient Method for Demethylation of Aryl Methyl Ether Using Aliquat-336”, Syn. Commun, 2013, 43, 3272-3280, DOI: 10.1080/00397911.2013.772201.
- Chae, J., “Practical Demethylation of Aryl Methyl Ethers using an Odorless Thiol Reagent”, Arch. Pharm. Res. 2008, 31, 305-309. DOI: 10.1007/s12272-001-1156-y.
Related Books
[amazonjs asin=”1118057481″ locale=”JP” title=”Greene’s Protective Groups in Organic Synthesis”]