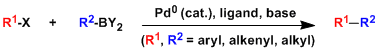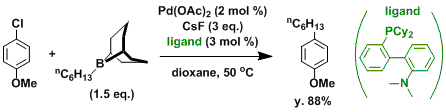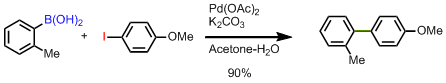- Popularity
- Responsibility
- user-friendliness
- Criteria #4
- Criteria #5
-
Characteristics
- A cross-coupling reaction between organoboron and organoboron and organohalide compounds via palladium catalysis, which was discovered by Akira Suzuki and Norio Miyaura (Hokkaido University, Japan) in 1979. A very useful reaction among the many palladium catalyzed coupling reactions, owing to its mild reaction conditions and high functional group selectivity.
- Although various organoboron reagents can be used in this reaction, organoboronic acids are paticularly suitable since they are easily synthesized, are stable to air and water, and crystaline. Moreover, boron-containing side products are nontoxic. These synthetic advantages and utilities permit this reaction to be developed into industrial scales.
- Reacently, many organoboronic acids are commercially available, and thus the synthesis of biaryl compounds has become quite a routine procedure. This cross-coupling reaction is useful not only for the synthesis of medicines and other materials used in research, but also for large-scale production of synthetic fibers and liquid crystals. Amang organic name reactions crowned with a Japanese name, it is one of the most popular and useful reactions owing to its wide range of applicability.
-
Literature reference
[1] Miyaura, N.; Suzuki, A. J. Chem. Soc., Chem. Commun. 1979, 866. DOI:10.1039/C39790000866
[2] Miyaura, N.; Yamada, K.; Suzuki, A. Tetrahedron Lett. 1979, 3437.
[3] Suzuki, A. Pure. Appl. Chem. 1985, 57, 1749.
[4] Review for Suzuki Coupling Reaction: Miyaura, N.; Suzuki, A. Chem. Rev.1995, 95, 2457. DOI:10.1021/cr00039a007
[5] Review for B-alkyl Suzuki Coupling Reaction: Danishefsky, S. J. et al. Angew. Chem. Int. Ed. 2001, 40, 4544.
-
Reaction mechanism
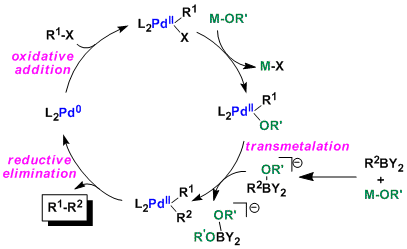
Generally, carbon-boron bonds are strong (organoboron compounds are stable in nature) and thus direct transmetalation from organobronic acids does not occur. To this end, an excess of base must be added to create organoborates, which are then amenable to transformation (this unfortunately results in the disadvantage of not being able to use base sensitive reactants).
-
Example of reactions
Modern methods in cross-coupling chemistry evolve so rapidly that even arylhalides with low reactivity, such as aryl chlorides, are now substates amenable to cross-coupling[6][7]
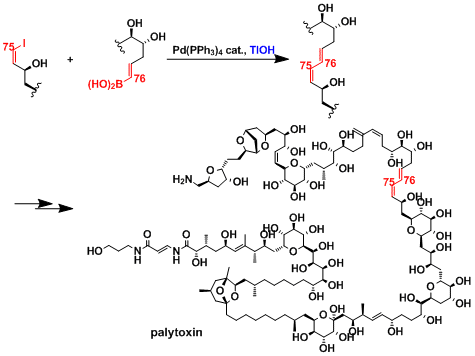
By making use of this reactions high functional group selectivity, the total synthesis of palytoxtin was achieved. Generally, cross-coupling reactions become more difficult as the molecular size is increaced, due to the decreased probability of the required functional groups to be in the vicinity of one another. To overcome this problem, Kishi et al. have utilzed thallium hydroxide as the base to dramatically increase the reaction rate[8]
It has been long known that alkyl borates are difficult substrates for this coupling reaction since transmetalation of Csp3 bonds is slow, and since b-hydride elimination readily occurs even when transmetalation is achieved. After numerous research efforts, catalysts designed to overcome these problems were discovered, thereby leading to an even greater applicability of the Suzuki reaction in constructing carbon frameworks of complex organic compounds[5]
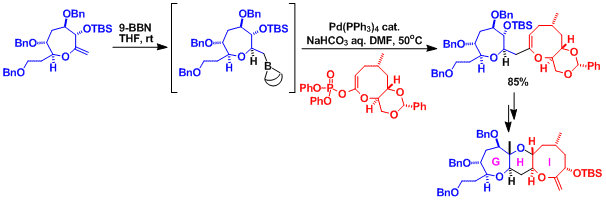
eg. Synthetic studies toward ciguatoxin[9]
An alkyl boron Suzuki cross-coupling was used in the convergent synthesis of the tricyclic core of ciguatoxin. The substrate with the leaving group was designed to be an enol phosphate rather than an enol triflate to achieve enhanced stability.
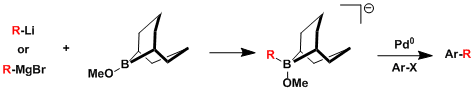
eg. Suzuki cross-coupling via boron-ate complexes[10]
A slight variant of the conventional Suzuki coupling, this reaction proceeds by pre-forming a bron-ate complex by mixing 9-BBN-OMe with an organolithium or Grignard reagent of choice. This becomes especially useful for installing methyl or alkynyl groups which are imppossible to install with the conventional hydroboration approach.
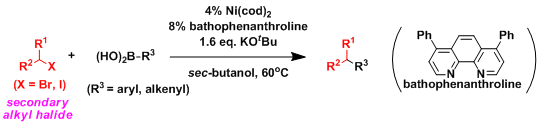 eg. Suzuki coupling with secondary alkyl halides[11]
eg. Suzuki coupling with secondary alkyl halides[11]
Commonly a metal center after oxidative addition bound to an alkyl group decomposes rapidly due to β-hydride ellimination. In the case of secondary alkyl halides, this is the main event happening in the reaction and hence there not been any successful examples. After an extensive screening process involving a nickel center and various ligands, Fu et al, have designed a catalyst that can achieve this dificult transformation.
-
Procedure
Accerated suzuki coupling via a ligandless palladium catalyst[12]
4-Methoxy-2′-methylbiphenyl . o-Tolylboronic acid, 10.0 g (73.6 mmol), 16.8 g (71.8 mmol) of 4-iodoanisole, and 200 mL of acetone are combined in a 1-L, three-necked flask equipped with an efficient stirbar, two stoppers, and a reflux condenser attached to a gas-flow adapter with a stopcock. Potassium carbonate, 25.0 g (0.180 mol), is dissolved in 200 mL of water in a separate 250-mL Schlenk flask. In a third flask (25-mL Schlenk flask) 3.30 mg (0.02 mmol, 0.2%) of palladium acetate is dissolved in 10 mL of acetone . All three flasks are then thoroughly degassed by four freeze-pump-thaw cycles. Under an argon back flow, one of the stoppers on the three-necked flask is replaced with a rubber septum, and the carbonate and catalyst solutions are added via cannula to form a biphasic mixture. The top layer turns brown upon addition of the catalyst. The septum is replaced with the glass stopper and three additional freeze-pump-thaw cycles are applied. The flask is then backfilled with argon, and the reaction is brought to reflux under a positive argon pressure. After 2 hr at reflux the heat source is removed and the reaction is allowed to cool. By this time the brown color has faded and the reaction is a triphasic mixture with copious amounts of palladium black floating between the layers. The reaction is transferred to a 1-L separatory funnel and extracted into diethyl ether (3 × 100 mL). The organic layers are combined, washed with water (1 × 100 mL) saturated with sodium chloride , and dried over magnesium sulfate . Solvent is removed with a rotary evaporator to yield a yellow oil which is distilled (125-130°C, 0.10 mm) to give 12.8 g of 4-methoxy-2′ methylbiphenyl as a colorless oil (90.3% yield)
-
Bibliography
[6] Fu, G. C. et al. Angew. Chem. Int. Ed. 1999, 37, 3387.
[7] Buchwald, S. L. et al. J. Am. Chem. Soc. 1999, 121, 9550.
[8] Kishi, Y. et al. J. Am. Chem. Soc. 1989, 111, 7530; ibid. 1994, 116, 11205.PDF
[9] Sasaki,M.; Tachibana, K. et al. Angew. Chem. Int. Ed. 2001, 40, 1090.
[10] Kalesse, M. ChemBioChem 2000, 1. 171.
[11] Fu, G. C. et al. J. Am. Chem. Soc. 2004, 126, 1340.
[12] Organic Syntheses, Coll. Vol. 10, p.501 (2004); Vol. 75, p.61 (1998).
- Related Books
[amazonjs asin=”B00EHKTLPY” locale=”US” title=”Organotrifluoroborate Preparation, Coupling and Hydrolysis (Springer Theses)”][amazonjs asin=”3527331549″ locale=”US” title=”Metal Catalyzed Cross-Coupling Reactions and More, 3 Volume Set”][amazonjs asin=”3642323677″ locale=”US” title=”Applied Cross-Coupling Reactions (Lecture Notes in Chemistry)”]
-
Related links
Organoborane (Wikipedia)
Suzuki Reaction (Wikipedia)