- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
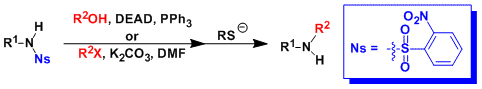
-The Fukuyama amine synthesis provides a powerful way to access secondary amines under mild reaction conditions.
-Nitrobenzenesulfonamides (nosyl (Ns) amides) are acidic enough to be alkylated under the Mitsunobu conditions. They can be alkylated by a weak base and an alkyl halide too.
-Nosyl group can be deprotected by aromatic nucleophilic substitution with thiols, which is the most important advantage over tosyl group.
-
General References
・Review: Kan, T.; Fukuyama, T. Chem. Commun. 2004, 353. DOI: 10.1039/b311203a
-
Reaction Mechanism
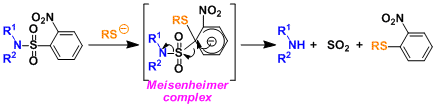
The deprotection of Ns involves the addition of a thiolate forming a Meisenheimer complex, followed by the elimination of sulfur dioxide and the free amine. This convenient deprotection is the unique feature of Ns group.
-
Examples
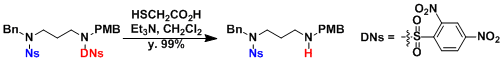
The dinitro version (DNs) that can be deprotected selectively in the presence of Ns is available too.
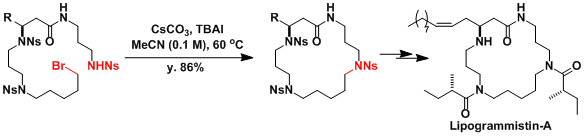
An application in the synthesis of a macrocyclic amine.[1]
-
Experimental Procedure
Deprotection of nosyl group.[2]
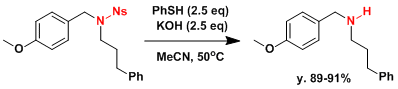
A 100-mL, two-necked, round-bottomed flask equipped with a magnetic stirring bar, nitrogen gas inlet, and a rubber septum is charged with 7.82 mL (76.5 mmol) of thiophenol and 20 mL of acetonitrile. The mixture is cooled in an ice-water bath and 10.9 M aqueous potassium hydroxide solution (7.02 mL, 76.5 mmol) is added over a period of 10 min. After 5 min, the ice-water bath is removed, and 13.5 g (30.6 mmol) of N-(4-Methoxybenzyl)-N-(3-phenylpropyl)-2-nitrobenzenesulfonamide in 20 mL of acetonitrile is added over 20 min. The reaction mixture is heated in a 50°C oil bath for 40 min. The reaction mixture is allowed to cool to room temperature, diluted with 80 mL of water, and extracted with three 80-mL portions of dichloromethane. The combined organic extracts are washed with brine (80 mL), dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The residue is purified by column chromatography on silica to give 7.81 g of the desired amine and its hydrochloride salt. This oil is dissolved in 120 mL of dichloromethane and washed with two 80-mL portions of 1 M aqueous sodium hydroxide solution, 40 mL of brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure. Bulb-to-bulb distillation (0.25 mm, oven temperature 150°C) provides 6.98-7.08 g (89-91%) of N-(4-Methoxybenzyl)-3-phenylpropylamine as a colorless oil.
-
Experimental Tips
-
References
[1] Fujiwara, A.; Kan, T.; Fukuyama, T. Synlett 2000, 1667. DOI: 10.1055/s-2000-7950
[2] Kurosawa, W.; Kan, T.; Fukuyama, T. Org. Synth. 2002, 79, 186. [PDF]
-
Related Books