Lummis, P. A.; Momeni, M. R.; Lui, M. W.; McDonald, R.;Ferguson, M. J.; Miskolzie, M.; Brown, A.; Rivard, E. Angew. Chem. Int. Ed. 53, 2014. 9347 – 9351.
DOI: 10.1002/anie.201404611
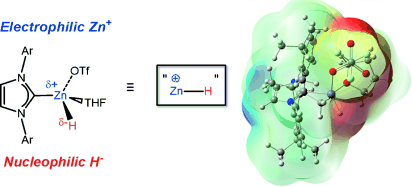
We present isolable examples of formal zinc hydride cations supported by N-heterocyclic carbene (NHC) donors, and investigate the dual electrophilic and nucleophilic (hydridic) character of the encapsulated [ZnH]+units by computational methods and preliminary hydrosilylation catalysis.
Zinc dihydride (ZnH2) which has been recognized since 1947, is pyrophoric, insoluble in most solvents, and gradually decomposes under ambient conditions. Meanwhile, the potential utility of ZnH2 is as a reducing agent. Indeed, ZnH2 supported by a judicious co-ligand becomes soluble and more thermally stable, with various nuclearity depending on the steric bulkiness as well as denticity of the ligands. It would be useful if zinc hydridecomplex could show a practical catalytic activity as it is inexpensive (~0.065 euro/mol), relatively abundant (0.0076% in the earth crust), environmentally benign, and low-toxic. 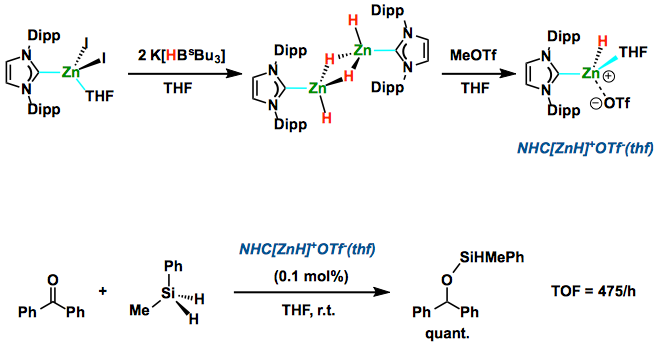 In order to increase the reactivity of zinc hydride complex, Brown and Rivard et al. proposed to generate a complex featuring formally cationic [ZnH]+moieties so as to open coordination sites for substrates binding/activation. They showed how to create [ZnH]+moieties concurrently retaining the requisite hydridic character, and demonstrated preliminary catalysis results. They synthesized NHC-stabilized [ZnH]+cations that are thermally stable but still active as catalysts for the hydrosilylation and hydroboration of ketones under mild conditions. Interestingly, NHC·ZnH(OTf)·THF is much more stable than [NHC·ZnH2]2, thus easy to handle for catalysis reactions. This work will apply to a wider range of substrates and transformation such as the reduction of CO2.
In order to increase the reactivity of zinc hydride complex, Brown and Rivard et al. proposed to generate a complex featuring formally cationic [ZnH]+moieties so as to open coordination sites for substrates binding/activation. They showed how to create [ZnH]+moieties concurrently retaining the requisite hydridic character, and demonstrated preliminary catalysis results. They synthesized NHC-stabilized [ZnH]+cations that are thermally stable but still active as catalysts for the hydrosilylation and hydroboration of ketones under mild conditions. Interestingly, NHC·ZnH(OTf)·THF is much more stable than [NHC·ZnH2]2, thus easy to handle for catalysis reactions. This work will apply to a wider range of substrates and transformation such as the reduction of CO2.  Related Links
Related Links