G, Copley.; J, Gillmore.; J, Crisman.; G, Kodis.; C, Gray.;B, Cherry.; B, Sherman.; P, Liddell.; M, Paquette.; L, Kelbauskas.; N, Frank.; A, Moore.; T, Moore.; D, Gust. J. Am. Chem. Soc., 2014, ASAP
DOI: 10.1021/ja504879p
Two molecules in which the intensity ofshorter-wavelength fluorescence from a strong fluorophore ismodulated by longer-wavelength irradiation of an attachedmerocyanine−spirooxazine reverse photochromic moiety havebeen synthesized and studied. This unusual fluorescencebehavior is the result of quenching of fluorophore fluorescenceby the thermally stable, open, zwitterionic form of thespirooxazine, whereas the photogenerated closed, spirocyclicform has no effect on the fluorophore excited state. Thepopulation ratio of the closed and open forms of thespirooxazine is controlled by the intensity of the longer-wavelength modulated light. Both square wave and sine wavemodulation were investigated. Because the merocyanine−spirooxazine is an unusual reverse photochrome with a thermally stable long-wavelength absorbing form and a short-wavelength absorbing photogenerated isomer with a very short lifetime, this phenomenon does not require irradiation of the molecules with potentially damaging ultraviolet light, and rapid modulation of fluorescence is possible. Molecules demonstrating these properties may be useful in fluorescent probes, as their use can discriminate between probe fluorescence and various types of adventitious “autofluorescence” from other molecules in the system being studied.
Introduction
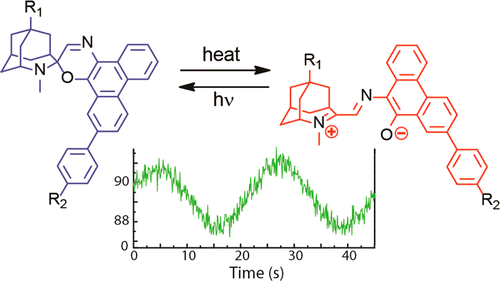
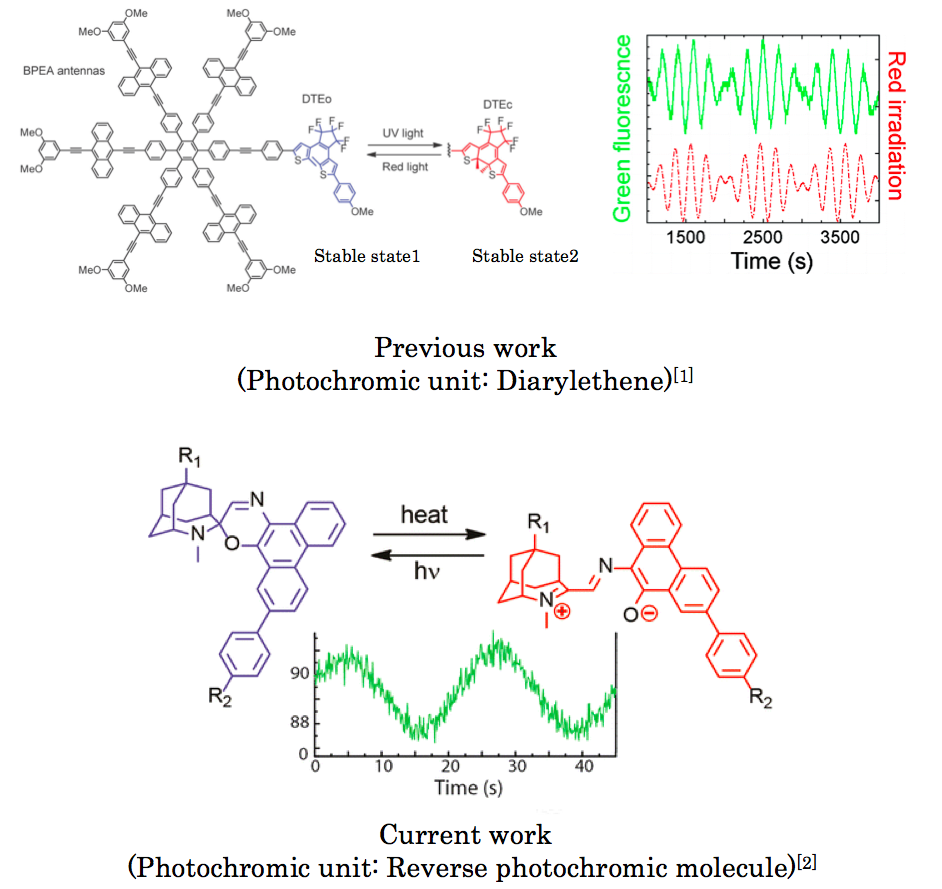
Photochromic molecule is specific group of molecule that upon photo-irradiation changes between its intrinsic two possible states, resulting observable change in colors. During past decades, fluorescent switchable photochromic molecule has been actively studied. However, the vast majority of them achieved the property by FRET or PET, which means that it is difficult to achieve rapid modulation of fluorescent intensity in long wavelength region.[1] The Gust group of present paper achieved this using reverse fluorescent photochromic molecule.[2]
Molecular design
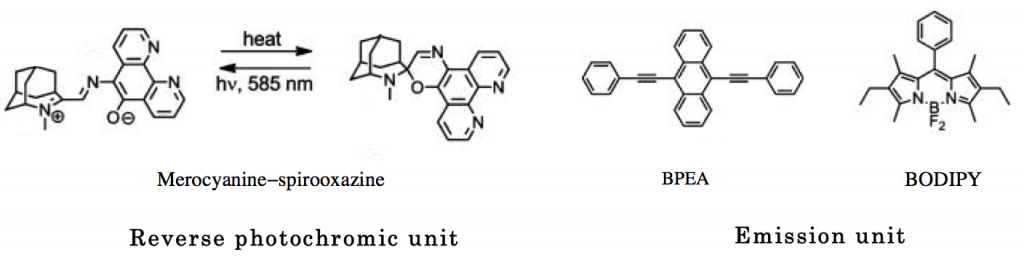
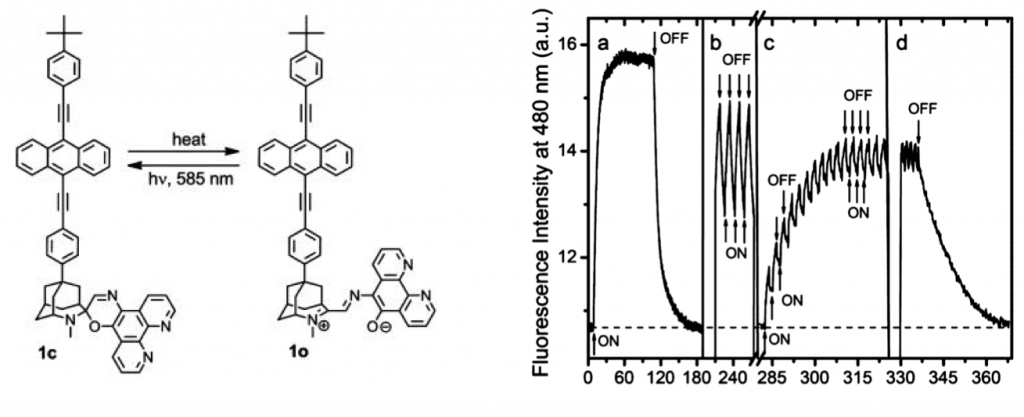
Three molecules which are composed of photochromic unit and emission unit as shown below.
(1) Although not widely known, can serve as reversible photochromic molecule. Unlike typical photochromic molecule has the stable initial form in longer region and unstable second form in shorter wavelength region.
(2) BPEA and BODIPY were used and connected to photochromic unit.
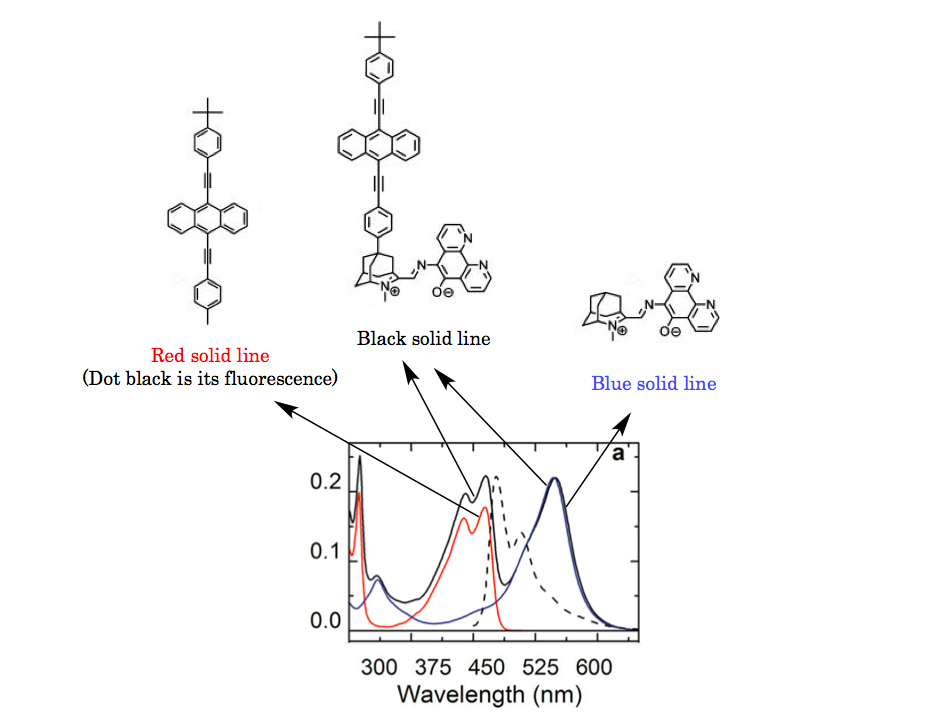
<Spectrum>
To shed light on the detail of complex spectrum, emission unit and photochromic unit are independently compared separately. It turned out that the obtained whole spectrum was result of liner combination of both units. One example was depicted as following.
Fluorescence modulation
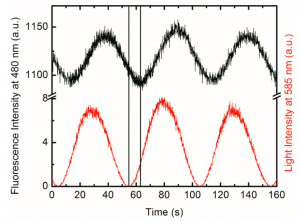
Upon 585 nm laser exposure to 1o, 1c having shorter wavelength absorption region was formed. Furthermore, BPEA’s blue fluorescence which have kept quenched got recovered at same time. The minute the irradiation was ceased, 1c rapidly went back to the initial 1o state. The fluorescence modulation was monitored with alternative 585 nm irradiation and time lapse, during which 440 nm light are constantly applied.
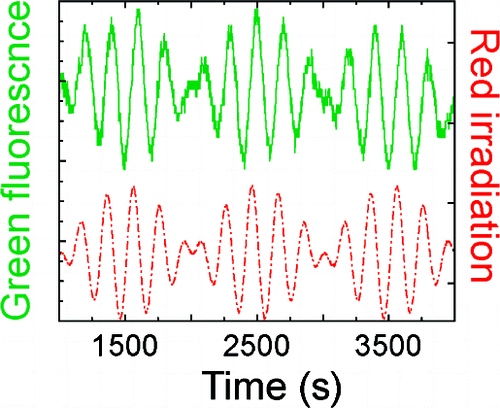
The power of photo-bleaching 585 nm was applied as sine carve intensity while second weak constant 440 nm excitation light were exposed at same time. Looking back at introduction, the obtained fluorescence modulation spectrum showed hundred-fold improvement compared to that of previous work.
References
A molecular “hexad” in which five bis(phenylethynyl)anthracene (BPEA) fluorophores and a dithienylethene photochrome are organized by a central hexaphenylbenzene unit has been prepared. Singlet−singlet energy transfer among the BPEA units occurs on the 0.4 and 60 ps time scales, and when the dithienylethene is in the open form, the BPEA units fluoresce in the 515 nm region with a quantum yield near unity. When the dithienylethene is photoisomerized by UV light to the closed form, which absorbs in the 500−700 nm region, the closed isomer strongly quenches all of the excited singlet states of BPEA via energy transfer, causing the fluorescence quantum yield to drop to near zero. This photochemical behavior permits the hexad to function in a manner analogous to a triode vacuum tube or transistor. When a solution of the hexad is irradiated with steady-state light at 350 nm and with red light (>610 nm) of modulated intensity, the BPEA fluorescence excited by the 350 nm light is modulated accordingly. The fluorescence corresponds to the output of a triode tube or transistor and the modulated red light to the grid signal of the tube or gate voltage of the transistor. Frequency modulation, amplitude modulation, and phase modulation are all observed. The unusual ability to modulate intense, shorter-wavelength fluorescence with longer-wavelength light could be useful for the detection of fluorescence from probe molecules without interference from other emitters in biomolecular or nanotechnological applications.
[2]” A Solution- and Solid-State Investigation of Medium Effects on Charge Separation in Metastable Photomerocyanines ”
Patel, D. G.; Paquette, M. M.; Kopelman, R. A.; Kaminsky, W.; Ferguson, M. J.; Frank, N. L. J. Am. Chem. Soc. 2010, 132, 12568− 12586.DOI: 10.1021/ja100238h
The effects of solution-state dielectric and intermolecular interactions on the degree of charge separation in metastable spirooxazine photomerocyanines (PMCs) is investigated. We report the first X-ray diffraction (XRD) analyses of an open form, a metastable photomerocyanine, of the spirooxazine class of photochromic molecules in two derivatives: spiro[azahomoadamantane-isoquinolinoxazine] (1) and spiro[azahomoadamantane-phenanthrolinoxazine] (2). Using the results of XRD analysis of the open photomerocyanine forms, in conjunction with computation, solvatochromism, and solution NMR studies, we have investigated the effect of the medium on the ground-state structure of these photomerocyanines. Solvatochromism and NMR chemical shift studies of 1 and 2 support the assignment of a quinoidal structure in nonpolar solvents and a zwitterionic structure in high-polarity solvents. The effect of azahomoadamantyl substitution is explored by comparing 1 and2 with the analogous indolyl derivatives, spiro[indoline-isoquinolinoxazine] (3) and spiro[indoline-phenanthrolinoxazine] (4) through XRD analysis of the closed spirooxazine (SO) forms, solution-state kinetic experiments, solvatochromism, and NMR studies. Longer Cspiro−O bond lengths in the SO form and slower rates of thermal PMC → SO isomerization for the azahomoadamantyl derivatives are associated with greater zwitterionic character in the PMC form, as found in the solvatochromism studies. XRD analysis of photomerocyanines 1 and 2indicate a greater contribution from the canonical zwitterionic resonance form relative to the quinoidal form in the solid state. Structural differences observed in two pseudopolymorphs of 2-PMC suggest that the degree of charge-separated character is influenced by the crystal packing environment. These results provide direct structural evidence for the effects of the medium polarity on charge-separated states of photomerocyanines.
Related Books
[amazonjs asin=”3527328467″ locale=”US” title=”Molecular Fluorescence: Principles and Applications”]