Tag Archives: total synthesis
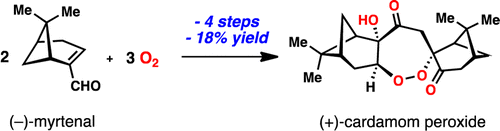
Four-Step Synthesis of the Antimalarial Cardamom Peroxide via an Oxygen Stitching Strategy
X. Hu, T. J. Maimone, J. Am. Chem. Soc. 2014, 136, 5287–5290. DOI:10.1021/ja502208z A four-step synthesis of the antimalarial terpene cardamom peroxide, a 1,2-dioxepane-containing natural product, is ...
Samuel J. Danishefsky
Samuel J. Danishefsky (born 1936, Bayonne, NJ) is synthetic organic chemist who accomplished in natural products total synthesis and pioneer for chemical synthesis of carbohydrates for the ...
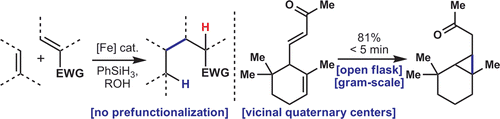
A Practical and Catalytic Reductive Olefin Coupling
Lo, J. C.; Yabe, Y.; Baran, P. S. J. Am. Chem. Soc. 2014, ASAP. DOI: 10.1021/ja4117632 A redox-economic method for the direct coupling of olefins that uses an inexpensive iron catalyst and a silane ...
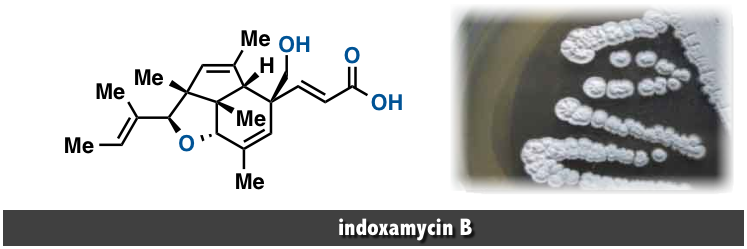
indoxamycin B
In 2009 a research group in Japan isolated a novel class of polyketides, subsequently named indoxamycins, from saline cultures of marine-derived actinomycetes. [1] Within this family, indoxamycins A ...
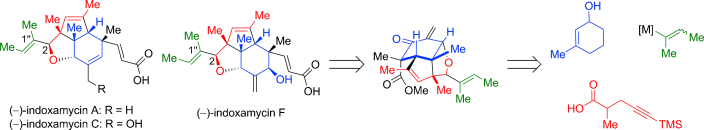
Divergent Total Synthesis of Indoxamycins A, C, and F
He, C.; Zhu, C.; Dai, Z.; Tseng, C.-C.; Ding, H. Angew. Chem. Int. Ed. 2013, 52, 13256–13260. DOI: 10.1002/anie.201307426 The concise and divergent total synthesis of (−)-indoxamycins A, C, and F has ...
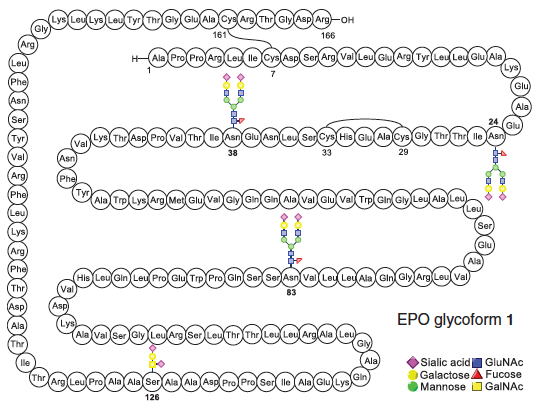
Erythropoietin Derived by Chemical Synthesis
Wang, P.; Dong, S.; Shieh, J.-H.; Peguero, E.; Hendrickson, R.; Moore, M. A. S.; Danishefsky, S. J. Science 2013, 342, 1357–1360. DOI: 10.1126/science.1245095 Erythropoietin is a signaling ...
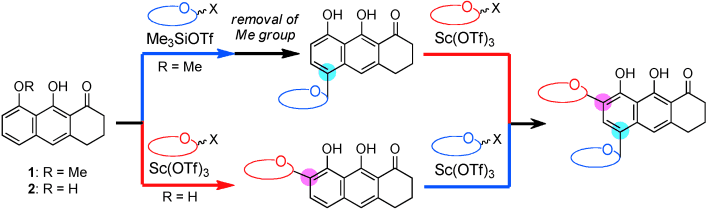
Synthesis of the Pluramycins 1: Two Designed Anthrones as Enabling Platforms for Flexible Bis-C-Glycosylation
Kitamura, K.; Ando, Y.; Matsumoto, T.; Suzuki, K. Angew. Chem. Int. Ed. 2013 Early View DOI: 10.1002/ange.201308016 Two effective tricyclic platforms are reported for the installation of the two ...
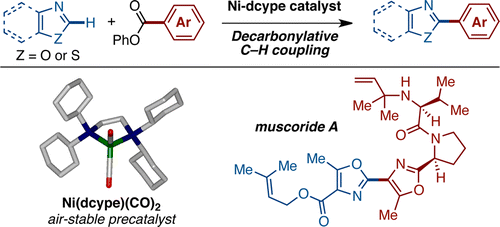
Decarbonylative C–H Coupling of Azoles and Aryl Esters: Unprecedented Nickel Catalysis and Application to the Synthesis of Muscoride A
Amaike, K.; Muto, K.; Yamaguchi, J.; Itami, K. J. Am. Chem. Soc., 2012, 134 , 13573–13576. DOI: 10.1021/ja306062c A nickel-catalyzed decarbonylative C–H biaryl coupling of azoles and aryl ...