X. Hu, T. J. Maimone, J. Am. Chem. Soc. 2014, 136, 5287–5290.
DOI:10.1021/ja502208z
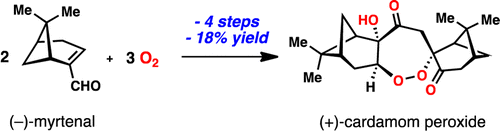
A four-step synthesis of the antimalarial terpene cardamom peroxide, a 1,2-dioxepane-containing natural product, is reported from (−)-myrtenal and molecular oxygen. This highly concise route was guided by biosynthetic logic and enabled by an unusual manganese-catalyzed, tandem hydroperoxidation reaction. The absolute configuration of the cardamom peroxide is reported, and its mode of fragmentation following Fe(II)-mediated endoperoxide reduction is established. These studies reveal the generation of reactive intermediates distinct from previously studied endoperoxide natural products.
Tom Maimone,[1] who is assistant professor on University of California, Berkeley reported the rapid synthesis of cardamom peroxide to J. Am. Chem. Soc. They described that cardamon peroxide can be synthesized form two pinenes adding 3.5 equiv oxygen, followed by the dehydration of one water. That is absolutely desired transformation, but I like this kind of approach that like the toward synthesis of quinine by William H. Perkin in 1856. In fact, they accomplished to synthesize it by following the 4 steps. 1) McMurry coupling of two pinene; 2) addition of molecular oxygen 3) Dess-Martin oxidation, and 4) unusual manganese-catalyzed, tandem hydroperoxidation reaction.
-
References
He got Ph.D in Phil Baran group at The Scripps Research Institute, and his representative papers during Ph.D are shown in here.
[1] “Total synthesis of marine natural products without using protecting groups”
P. S. Baran, T. J. Maimone, J. M. Richter, Nature 2007, 446, 404. DOI: 10.1038/445826a
[2] “Total Synthesis of Vinigrol”
T. J. Maimone, J. Shi, S. Ashida, P. S. Baran, J Am Chem Soc 2009, 131, 17066. DOI: 10.1021/ja908194b
-
Related Books
[amazonjs asin=”3642340644″ locale=”US” title=”Total Synthesis of Natural Products: At the Frontiers of Organic Chemistry”]
- Related Link