- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
-The stereoselective aldol reaction in which the nucleophiles bearing an oxazolidinone chiral auxiliary are reacted with the aldehydes is called the Evans aldol reaction. Chiral auxiliaries derived from phenylalanine and valine are the most popular.
-The reaction requires a stoichiometric chiral source and the steps to prepare/remove the chiral auxiliary, but the benefit of reliable stereocontrol usually outweighs the demerits. The “Evans-syn” conditions that use boron triflate is particularly useful to form carbon-carbon bonds with almost perfect and exception-less stereoselectivity. The reaction is amenable to large scale applications and often used in early stages of synthesis.
-The Evans aldol reaction has been used as a standard strategy in the synthesis of natural products, particularly polyketide compounds.
-
General References
・Evans, D. A.; Vogel, E.; Nelson, J. V. J. Am. Chem. Soc. 1979, 101, 6120. DOI: 10.1021/ja00514a045
・Evans, D. A.; Bartroli, J.; Shih, T. L. J. Am. Chem. Soc. 1981, 103, 2127. DOI: 10.1021/ja00398a058
・Evans, D. A. Aldrichimica Acta 1982, 15, 23.
・Kim, B. M. et al. Comp. Org. Syn. 1991, 2, 239.
・Review: Evans, D. A. et al. Actualité Chimique, 2003, 35.
-
Reaction Mechanism
The imide activated by boron triflate is deprotonated by triethylamine at the α-position to form a Z-enolate. The enolate reacts with the aldehyde through six-membered chair transition state to give the syn product (Evans-syn). The chiral auxiliary is thought to assume the conformation shown in the brackets in the picture in order to minimize the dipole repulsion between the carbonyl groups. (ref: J. Am. Chem. Soc. 1981, 103, 3099.)
-
Examples
The Evans aldol products of all possible stereochemistry have been synthesized by choosing the combination between the Lewis acid and the chiral auxiliary.[1,2,3] The stereoselectivities are explained by the differences in the shape of the transition state. The conformation of the transition state depends on the type of auxiliary and the coordination number (chelating property) of the Lewis acidic metal.
The oxazolidinone auxiliary can be converted into various functional groups. The direct conversion into the Weinreb amide is especially useful.
-
Experimental Procedure
-
Experimental Tips
-
References
[1] “Non-Evans Syn”: (a) Crimmins M. T., King B. W., Tabet A. E. J. Am. Chem. Soc. 1997, 119, 7883. doi:10.1021/ja9716721 (b) Crimmins M. T., Chaudhary K. Org. Lett. 2000, 2, 775. doi:10.1021/ol9913901 (c) Crimmins M.T.; King B.W.; Tabet E.A.; Chaudhary K. J. Org. Chem. 2001, 66, 894. doi: 10.1021/jo001387r
[2] “Non-Evans Anti” & “Evans Anti”: Evans D.A.; Tedrow J.S.; Shaw J.T.; Downey C.W.; J. Am. Chem. Soc. 2002, 124, 392. doi: 10.1021/ja0119548
-
Related Books
[amazonjs asin=”3527307141″ locale=”US” title=”Modern Aldol Reactions (2 Volume Set)”]

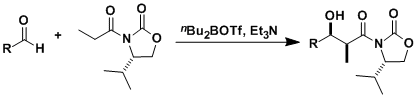
![evans_aldol_2[1]](https://assets.en.chem-station.com/uploads/2014/04/evans_aldol_21.gif)
![evans_aldol_3[1]](https://assets.en.chem-station.com/uploads/2014/04/evans_aldol_31.gif)
![evans_aldol_4[1]](https://assets.en.chem-station.com/uploads/2014/04/evans_aldol_41.gif)