- Generality
- Reagent Availabiltiy
- Historical Significance
- Criteria #4
- Criteria #5
-
General Characteristics
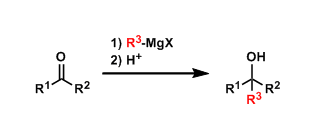
Grignard reagents are the most popular among organometallic reagents. Alkyl groups can be introduced into carbonyl compounds to give the corresponding alcohols.
The introduction of alkyl groups with β-hydrogen leads to by-products of hydride reduction of ketones.
A reaction of magnesium with a mixture of alkyl halides and carbonyl compounds to give the alcohol is called the Barbier reaction. In this case, metals other than magnesium, such as lithium, zinc, and samarium, can be used.
Different names are known depending on the reaction substrate: Berary reaction for β-amino-α,β-unsaturated ketone; Bodoroux-Chichibabin aldehyde synthesis reaction for orthoesters; the Bouveault aldehyde synthesis for formylamides.
V. Grignard, the developer of this reaction, was awarded the Nobel Prize in Chemistry in 1912
-
General References
- Grignard, V. Compt. Rend. 1900, 130, 1322.
Primitive Review:
- Shirley, D. A. Org. React. 1954, 8, 28.
- Huryn, D. M. Comprehensive Organic Synthesis 1991, 1, 49.
Recent Progress & Reviews:
- Franzen, R. G. Tetrahedron 2000, 56, 685. doi:10.1016/S0040-4020(99)00963-1
- Hoffmann, R. W. Chem. Soc. Rev. 2003, 32, 225. doi: 10.1039/b300840c
- Garst, J. F.; Soriaga, M. P. Coord. Chem. Rev. 2004, 248, 623. doi:10.1016/j.ccr.2004.02.018
- Seyferth, D.;Organometallics 2009, 28, 1598. DOI: 10.1021/om900088z
-
History
Dialkyl zinc has a longer history as an addition reactant for carbonyl compounds. However, its low reactivity and pyrophoric properties have been problematic. In 1900, French chemist Victor Grignard discovered that the reaction of alkyl halides with magnesium in ether solvent yielded very reproducible and highly reactive alkylating agents. This became the Grignard reagent and is used to this day.
-
Reaction Mechanism
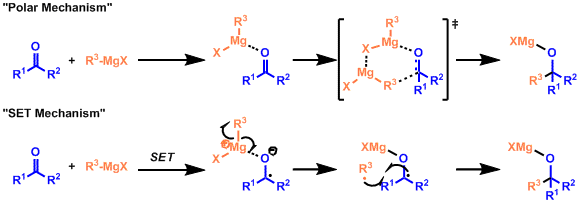
Grignard reagents form various complexes in solution via Schlenk equilibrium.
![]()
Magnesium serves as a Lewis acid for carbonyl compounds, and the nucleophilic addition of alkyl groups to the activated carbonyl compounds proceeds (Polar Mechanism). Easily reducible substrates such as benzophenone or bulky reagents such as t-Bu Grignard reagent undergo the Grignard reaction with a one-electron transfer mechanism (SET Mechanism).
-
Examples
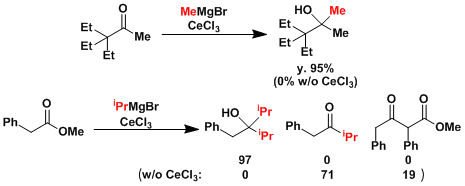
- When reacted in the presence of anhydrous cerium trichloride, side reactions such as enolization, reduction, and 1,4-addition are suppressed, and the expected adduct, alcohol, forms in good yield.[1]
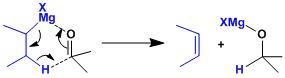
- i-PrMgBr often undergoes β-hydride reduction. An alternative method is the Grignard reaction with isopropenyl magnesium bromide followed by hydrogenation of olefins.
- Organomagnesiumate complexes show better chemoselectivity over RMgX.[2]
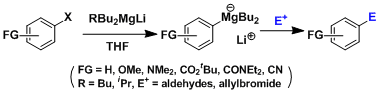
- Reactions with nitriles are useful for carbonyl synthesis.
![]()
- Chiral Grignard reagents are available.[3]
- Halogen-metal exchange with i-PrMgX gives functionalized Grignard reagents under mild conditions. Addition of LiCl is effective (Turbo Grignard reagents).[4]
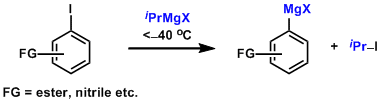
- The addition of zinc chloride is effective for selective alkylation over β-hydride reduction.[5]
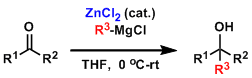
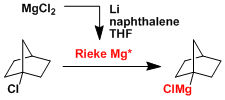
- Rieke magnesium gives Grignard reagents from unreactive alkyl halides.[6]
-
Experimental Procedure
-
Experimental Tips
- Simple Grignard reagents are commercially available. The reagent is likely to crystallize and precipitate if refrigerated or concentrated. So, it should be stored at an appropriate concentration or temperature so that the precipitates are completely dissolved before use.
- If the magnesium surface is inactivated, the Grignard reagent is less likely to form. Surface activation methods include a dilute hydrochloric acid wash or grind in a Glovebox (especially useful for small scales).
- The addition of a very small amount of iodine or dibromoethane or diiodoethane with Mg often promotes the formation of the Grignard reagent, and for substrates that do not readily form the Grignard reagent, heating in a flask equipped with a gymnolot is usually used. Since the reaction is exothermic, and once the formation starts, the reaction proceeds autocatalytic. Be careful not to runaway!
- The reaction is usually prepared with THF or ether as the solvent. The coordinating solvent stabilizes the Grignard reagent and promotes its formation. Only in certain cases, only ether is used (e.g., Mg+MeI will yield only Wurtz homocouplings if you try to prepare it in THF). Preparing allyl Grignard reagents requires skill and patience, including long addition periods and strict low-temperature control, because homo-coupling reactions easily compete.
- Titration method: add the Grignard reagent to an anhydrous methanol solution of 1,10-phenanthroline. Inexpensive solid alcohols such as menthol are also easy to use. The titration is complete when it turns reddish-purple in color. The principle is the formation of a phenanthroline-magnesium complex. [7]

Addition of Girgnard reaction to 1,10-phen and menthol in THF. Titration is finished when the red-purple color persists.
-
References
- (a) Imamoto, T. et al. J. Am. Chem. Soc. 1989, 111, 4392. DOI: 10.1021/ja00194a037 (b) Takeda, N.; Imamoto, T. Org. Synth. 1998, 76, 228. DOI: 10.15227/orgsyn.076.0228
- Ohshima, K. et.al Angew. Chem. Int. Ed. 2000, 39, 2481. [abstract]
- Hoffmann, R. W. et al. Angew. Chem. Int. Ed. 2000, 39, 3073. [abstract]
- Knochel, P. et al. Angew. Chem.Int. Ed. 2004, 43, 3333. DOI: 10.1002/anie.200454084
- Ishihara, K. et al. J. Am. Chem. Soc. 2006, 128, 9998. DOI: 10.1021/ja0628405
- (a) Rieke, R. D.; Bales, S. E.; Hudnall, P. M.; Burns, T. P.; Poindexter, G. S. Org. Synth. Coll. Vol. VI, 1988, 845. (b) Sell, M. S.; Klein, W. R.; Rieke, R. D. J. Org. Chem. 1995, 60, 1077. DOI: 10.1021/jo00109a048
- (a) Lin, H.-S.; Paquette, L. A. Synth. Commun.1994, 24, 2503. DOI: 10.1080/00397919408010560 (b) Walson, S. C.; Eastman, J. F. J. Organomet. Chem. 1967, 9, 165. DOI: 10.1016/S0022-328X(00)92418-5
-
Related Reactions
-
Related Media
-
Related Books
[amazonjs asin=”0127309454″ locale=”JP” title=”Organomagnesium Methods in Organic Chemistry (Best Synthetic Methods)”][amazonjs asin=”0824795458″ locale=”JP” title=”Handbook of Grignard Reagents (Chemical Industries)”][amazonjs asin=”0471999083″ locale=”JP” title=”Grignard Reagents: New Developments”]
-
External Links
- Victor Grignard (Wikipedia)
- Grignard Reagent (Wikipedia)
- Grignard Reaction/Grignard Reagent (organic-chemistry.org)
- Rieke Metals(Wikipedia)
- An introduction to Grignard reagents
- Grignard Reagents
- Japanese version of this article.