- Generality
- Reagent Availability
- Experimental User Friendliness
- Historical Significance
- Criteria #5
-
General Characteristics
The palladium- or nickel-catalyzed cross coupling between aryl halides/triflates and Grignard reagents is known as the Kumada-Tamao-Corriu reaction.
The Kumada-Tamao-Corriu reaction was one of the first sp2 carbon-carbon bond forming “cross coupling” reactions. Their pioneering work is regarded as an important milestone from both academic and practical perspectives.
The reaction procedure is simple and the reaction can be catalyzed by inexpensive nickel. Palladium catalysis tends to show more controlled reactivity and better chemoselectivity.
However, the Grignard reagents are inevitably too reactive to be used for compounds containing electrophilic and acidic functional groups. The convertion of Grignard reagents into less reactive organozinc reagents by transmetallation (the Negishi coupling) and the iron(III)-catalyzed coupling reactions (Kochi-Fürstner coupling) are effective complementary strategies.
-
General References
・Tamao, K.; Sumitani, K.; Kumada, M. J. Am. Chem. Soc. 1972, 94, 4374. DOI: 10.1021/ja00767a075
・Corriu, R. J. P.; Massse, J. P. J. Chem. Soc., Chem. Commun. 1972, 144. DOI: 10.1039/C3972000144a
・Yamamura, M.; Moritani, I.; Murahashi, S. J. Organomet. Chem. 1975, 91, C39. DOI: 10.1016/S0022-328X(00)89636-9
・Tamao, K.; Kumada, M. et al. Bull, Chem. Soc. Jpn. 1976. 49, 1958. doi:10.1246/bcsj.49.1958
-
Reaction Mechanism
The homocoupling of the Grignard reagent generates Ni(0) or Pd(0). The basic catalytic cycle consists of the oxidative addition of the aryl halide/triflate, transmetallation with the Grignard reagent, and reductive elimination.
-
Examples
An example of Ni-catalyzed asymmetric Kumada coupling.[1]
The Kumada coupling using the magnesium ate complex provides better functional group tolerance.[2]
An example of Ni-catalyzed Kumada coupling of aryl fluorides.[3]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Hayashi, T.; Hayashizaki, K.; Kiyoi, T.; Ito, Y. J. Am. Chem. Soc. 1988, 110, 8153. DOI: 10.1021/ja00232a030
[2] Martin, R.; Buchwald, S. L. J. Am. Chem. Soc. 2007, 129, 3844. doi:10.1021/ja070830d
[3] Yoshikai, N.; Mashima, H.; Nakamura, E. J. Am. Chem. Soc. 2005, 127, 17978. DOI: 10.1021/ja056327n
-
Related Books
[amazonjs asin=”3527305181″ locale=”US” title=”Metal-Catalyzed Cross-Coupling Reactions (2 Volume Set)”]
[amazonjs asin=”3527319379″ locale=”US” title=”Modern Arylation Methods”]
[amazonjs asin=”0470850329″ locale=”US” title=”Palladium Reagents and Catalysts: New Perspectives for the 21st Century”]
[amazonjs asin=”3540239820″ locale=”US” title=”Palladium in Organic Synthesis (Topics in Organometallic Chemistry)”]
[amazonjs asin=”3540421750″ locale=”US” title=”Cross-Coupling Reactions: A Practical Guide (Topics in Current Chemistry)”]

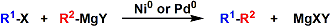
![kumada_tamao_2[1]](https://assets.en.chem-station.com/uploads/2014/05/kumada_tamao_21.gif)
![kumada_tamao_3[1]](https://assets.en.chem-station.com/uploads/2014/05/kumada_tamao_31.gif)
![kumada_tamao_4[1]](https://assets.en.chem-station.com/uploads/2014/05/kumada_tamao_41.gif)
![kumada_tamao_5[1]](https://assets.en.chem-station.com/uploads/2014/05/kumada_tamao_51.gif)