A heavy drug is a drug labeled with deuterium (2H, D).[1]
By replacing C-H bonds at metabolic sites in drug molecules with more stable C-D bonds, metabolism can be slowed down. This deuteration makes the drugs more stable and active for longer time. Therefore, one can reduce the number of pills per day, and side effects can be minimized.
Active ingredients of medicines are gradually decomposed by the liver and other organs, and eventually their pharmacological effects diminish. Since the degradation of drugs in the liver varies from person to person, the dosage may need to be adjusted for each patient to control the side effects. One strategy to solve these problems is the deuteration of pharmaceuticals.
The carbon-deuterium bonds (C-D bonds) in deuterated organic materials are stronger than the C-H bonds in non-deuterated compounds. Therefore, if the C-D bond is introduced into the metabolic site, pharmacological effect will last longer than non-deuterated analogs. In addition, deuteration is not simply used to slow down metabolism but is also used as a strategy to alter metabolic pathways.
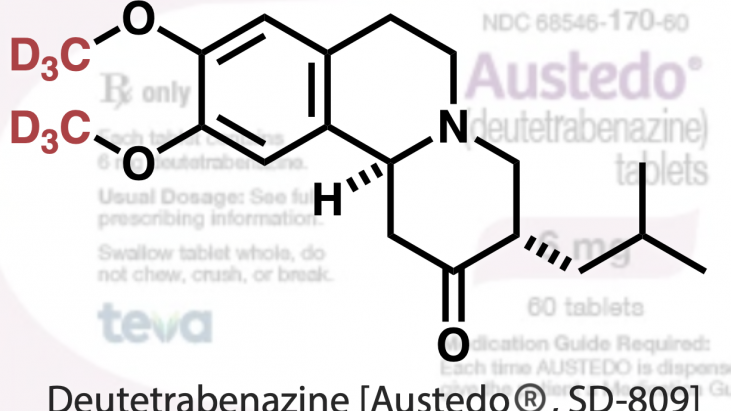
In 2017,a deuterated drug called deutetrabenazine (inhibition of involuntary movements in Huntington’s chorea) was first approved by the US Food and Drug Administration (FDA) (Figure 1). [2] This drug is a deuterated version of tetrabenazine.
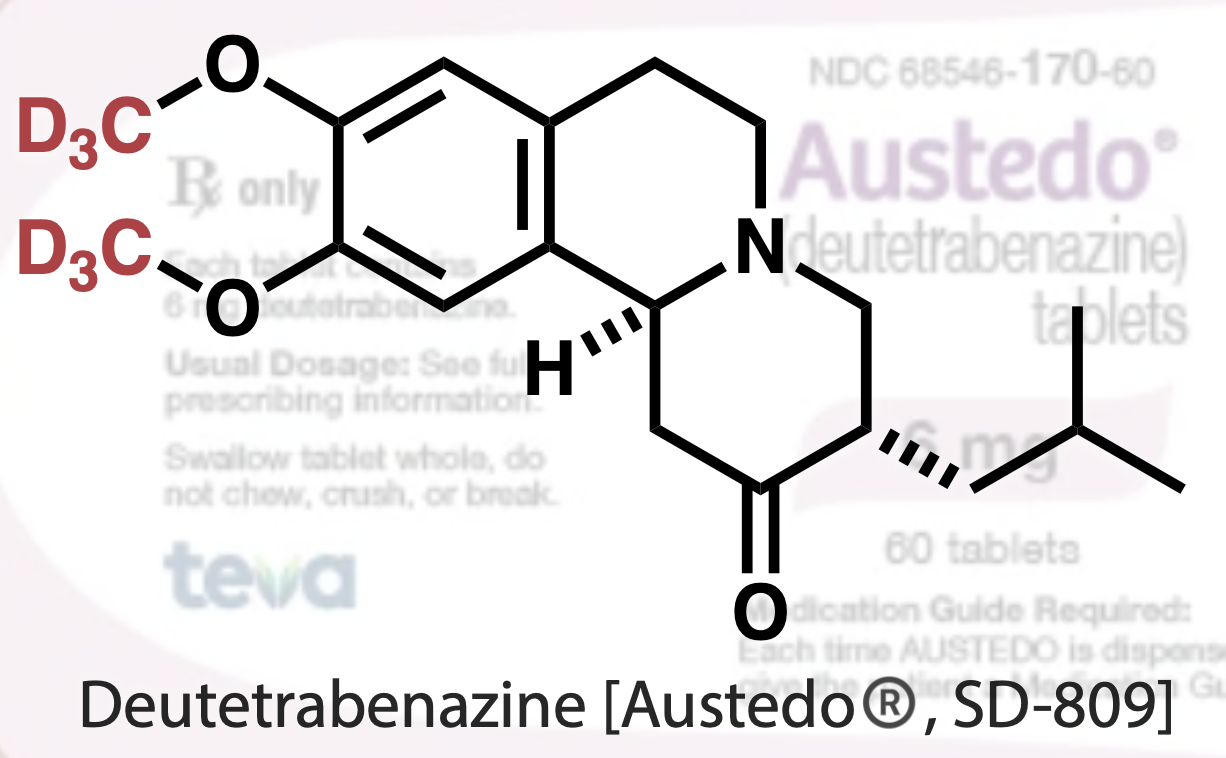
Figure 1
Deutetrabenazine is first metabolized at the carbonyl group to give an alcohol (Figure 2, left to middle). This alcohol is the active metabolite. Next, the methoxy group is demethylated by cytochrome P450 (CYP 2D6) (Figure 2, middle to right). Since both demethylated products are inactive, this demethylation results in the loss of drug efficacy.
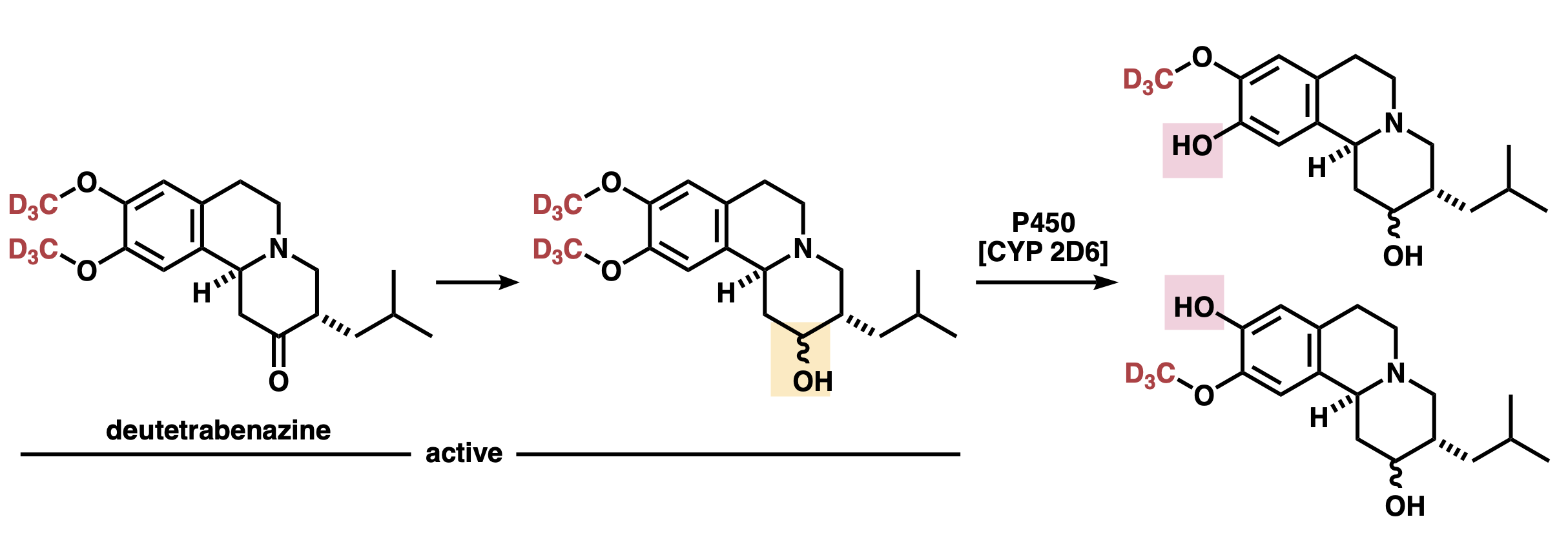
Figure 2
Demethylation by P450 proceeds with hydrogen abstraction. Deuteration of the methyl groups slows down this demethylation by kinetic deuterium isotope effect (KDIE). For an example of KDIE in demethylation, in the demethylation of anisole (methoxybenzene) by hepatic microsomes (P450), the demethylation of deuterated anisole in the lower equation was reported to be 5-8 times slower than in the upper equation in Figure 3. [3]
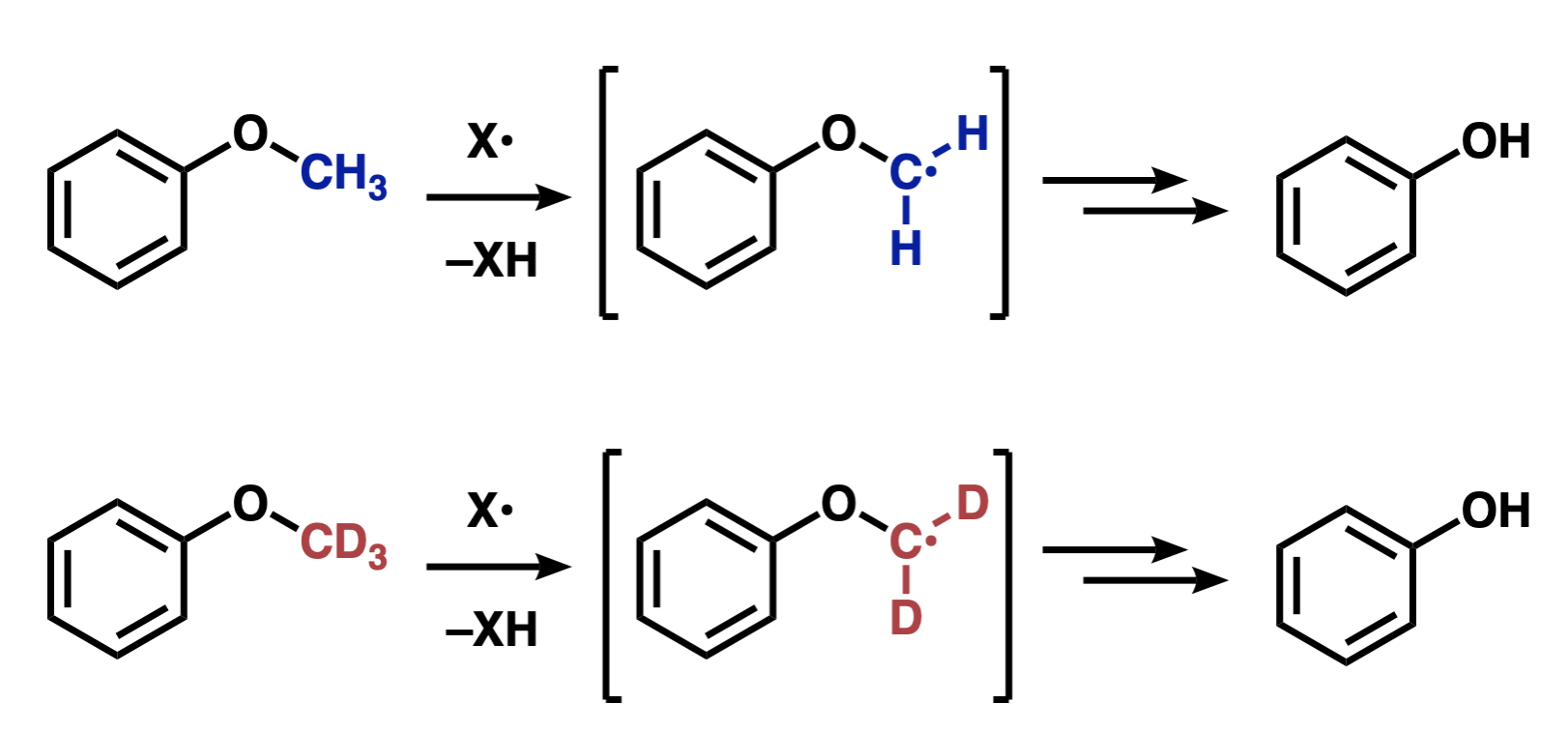
Figure 3
In 2021, the heavy drug donafenib was approved in China (anticancer drug, kinase inhibitor, Figure 4).[4] . As of 2022, there are several heavy drugs that are in clinical trials in various countries.
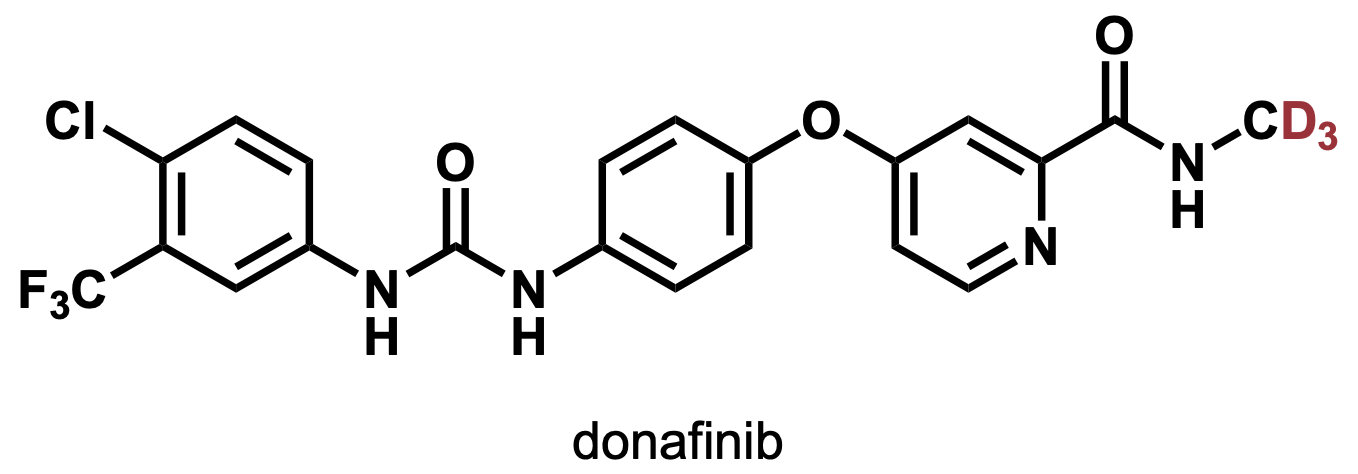
Figure 4
References
-
Pirali, T.; Serafini, M.; Cargnin, S.; Genazzani, A. A. Applications of Deuterium in Medicinal Chemistry. J. Med. Chem. 2019, 62, 5276−5297. DOI:10.1021/acs.jmedchem.8b01808
-
Dean, M.; Sung, V. W. Review of Deutetrabenazine: a Novel Treatment for Chorea Associated with Huntington’s Disease. Drug Des. Devel. Ther. 2018, 12, 313−319. DOI:10.2147/DDDT.S138828
-
Smith, J. R. L.; Sleath, P. R. Model systems for cytochrome P450 dependent mono-oxygenases. Part 2. Kinetic isotope effects for the oxidative demethylation of anisole and [Me-2H3]anisole by cytochrome P450 dependent mono-oxygenases and model systems. JCS Perkin Trans. II 1983, 621–628. DOI: 10.1039/P29830000621
-
Qin, S. et al. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J. Clin. Oncol. 2021, 39, 3002−3011. DOI: 10.1200/JCO.21.00163