Yue Wu, Yongshu Xie, Qiong Zhang, He Tian, Weihong Zhu, and Alexander D. Q. Li, Angew. Chem. Int. Ed. 2014, 53, 2090.
DOI: 10.1002/anie.201309915
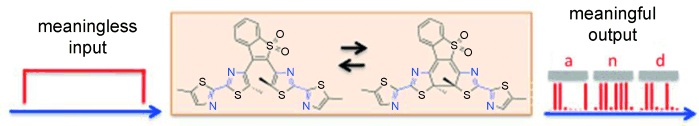
Using one ray of light to encode another ray of light is highly desirable because information in optical format can be directly transferred from one beam to another without converting back to the electronic format. One key medium to accomplish such an amazing task is photoswitchable mole- cules. Using bis(dithiazole)ethene that can be photoswitched between its ring-open and ring-closed states quantitatively with excellent fatigue resistance and high thermal stability, it is shown that quantitative photoreversibility allowed the photo- switching light to control other light travelling through the photoswitchable medium, a phenomenon of transferring information encoded in one light ray to others, thus imparting photo-optical modulation on the orthogonal light beam.
Introduction
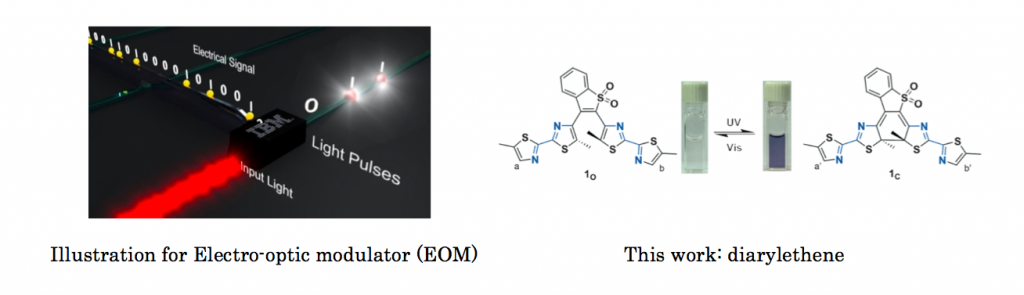
Electro-optic modulator (EOM) is an optical device in which a signal-controlled element exhibiting the electro-optic effect is used to modulate a beam of light.[1] Photochromic molecule which has high thermal stability and efficient conversion between two isomers can actually be utilized in (EOM) device. Professor Alexander at Washington State University for the first time demonstrated this concept using photochromic Diarylethene.
Molecular design
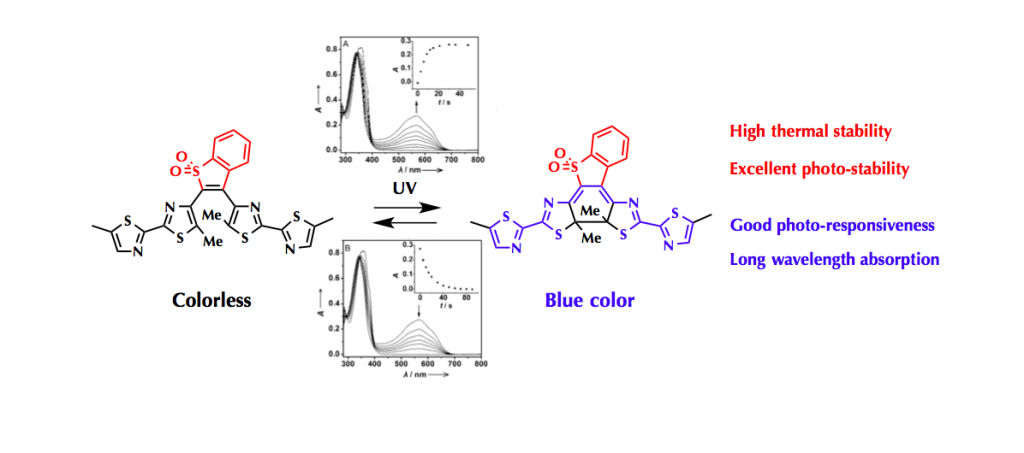
The diarylethen derivatives was design based on following strategies.
(1) Usual perfluorocyclopentene which is called head part was replaced with benzothiophene sulfone to increase photo stabilities.[2]
(2) Four thiazoles were adopted to achieve long wavelengths absorption in the closed form without decreasing the photo-responsiveness.[3]
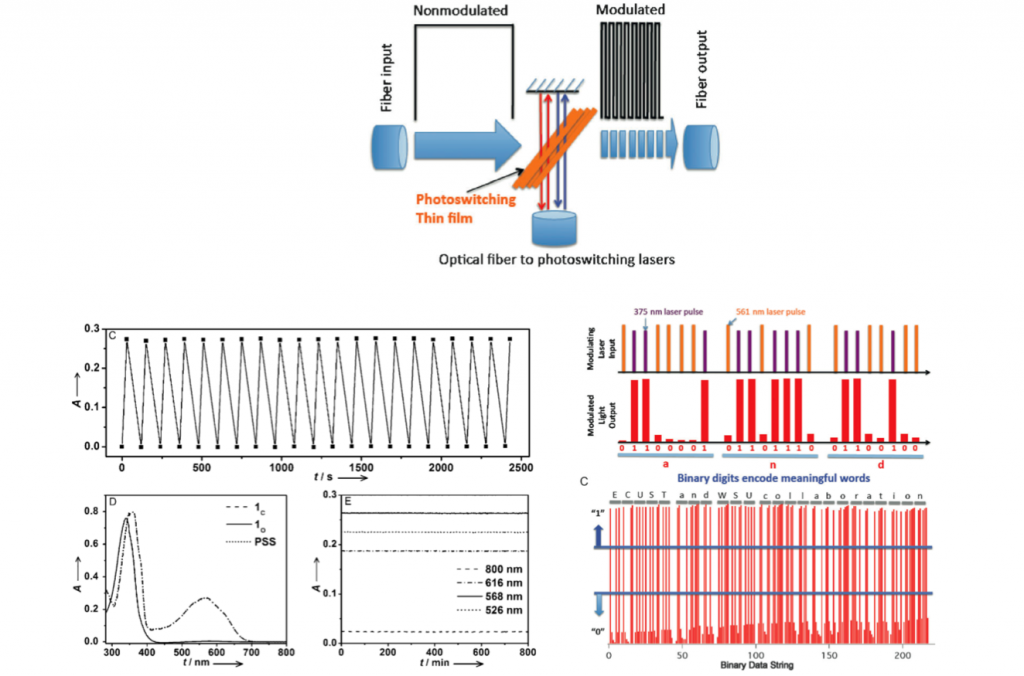
EOM demonstration
Compound 1 was fabricated in thin film. As shown below, three incident lights were set up to demonstrate optic modulator. Thanks to the excellent characteristics of diarylethene, the input light was successfully converted to meaningful information that can be defined either as 0 or 1. Authors further practiced this procedure on standard 8-bit ASCII characters and successfully extracted readable letters from output binary information.
Reference
[1]”Low (Sub-1-volt) halfwave voltage polymeric electro-optic modulators achieved by controlling chromophore shape”
Y. Q. Shi, C. Zhang, H. Zhang, J. H. Bechtel, L. R. Dalton, B. H. Robinson, W. H. Steier. Science, 2000, 288, 119. DOI: 10.1126/science.288.5463.119
[2]” The considerable photostability improvement of photochromic terarylene by sulfone group”
Yong-Chul Jeong, Chunji Gao, In Su Lee, Sung Ik Yang, Kwang-Hyun Ahn, Tetrahedron Lett, 2009, 50, 5288. DOI: 10.1016/j.tetlet.2009.07.028
Photochromic terarylene derivative 4 which has a sulfone group at the upper benzothiophene ring is readily synthesized using Suzuki coupling reaction. It exhibits good photochromic properties. Interestingly, the closed form of the compound 4 shows a good photostability as well as a thermal stability compared with its reduced analog 3, that provides a method to enhance the photostability of versatile photochromic terarylenes under UV.
[3]”Photon-Quantitative Reaction of a Dithiazolylarylene in Solution”
Fukumoto, S.; Nakashima, T; Kawai, T J. Am. Chem. Soc., 2012, 134, 19877. DOI: 10.1002/anie.201006844
Electrochemical response of photochromic tearylenes was surveyed by means of cyclic voltammetry, DFT calculations, and spectroelectrochemistry. 4,5-Bis(2-phenyl-5-methylthiazolyl)-2-phenylthiazole was found to show electrochemical oxidative ring-cycloreversion reaction. The net current efficiency of the cycloreversion reaction under constant potential electrolysis was as high as 900%, which is ascribed to an electrochemical local-cell mechanism and a chain reaction mechanism. Electron transfer stopped-flow study using a chemical oxidant successfully identified radical cation intermediates of both closed- and open-ring isomers, involved in the oxidative cycloreversion process. The significantly long-lived radical cation of open-ring isomer with the lifetime of 33 s takes part in the indirect electron transfer process from the neutral closed-ring isomer to the radical cation of open-ring isomer in the chain reaction manner.