- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The rearrangement of the carbenes formed by elimination of nitrogen from diazoketones giving ketenes is known as the Wolff rearrangement. The ketenes are trapped by nucleophiles in the system (water, alcohols, etc.) to give carboxylic acids and esters. The Wolff rearrangement of cyclic diazoketones is a useful ring contraction reaction.
When the diazoketones are synthesized from acid chlorides and diazomethane, the sequence leading to the rearrangement is particularly called the Arndt-Eistert synthesis, which is a useful way to effect one-carbon homologation of carboxylic acids.
-
General References
・Wolff, L. Liebigs Ann. Chem. 1902, 325, 129.
・Wolff, L. Ann.1912, 394, 25.
・Meier, H.; Zeller, K.-P. Angew. Chem. Int. Ed. Engl. 1975, 14, 32. doi:10.1002/anie.197500321
・Torres, M.; Lown, W. M.; Gunning, H. E.; Strausz, O. P. Pure Appl. Chem. 1980, 52, 1623. doi:10.1351/pac198052061623
・Gill, G. B. Comprehensive Organic Synthesis 1991, 3, 887.
・Yogev. A.; Loewenstein, R. M. J.; Amar, D. Chem. Rev. 1994, 94, 1091. DOI: 10.1021/ja00759a010
・Kirmse, W. Eur. J. Org. Chem. 2002, 2193. [abstract]
-
Reaction Mechanism
The generic mechanism involves the formation and the rearrangement of the α-ketocarbene intermediate. (Ref: J. Am. Chem. Soc. 1996, 118, 1551; J. Am. Chem. Soc. 1999, 121, 5930.)
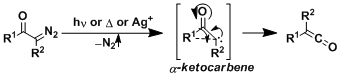
-
Examples
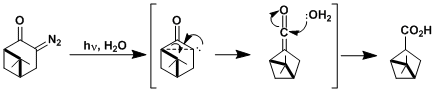
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Books

