- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
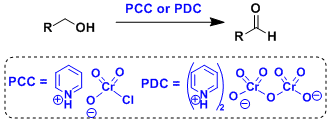
The oxidation of alcohols by PCC (Pyridinium Chlorochromate) or PDC (Pyridinium Dichromate) works under mild conditions and can be used for compounds containing unstable functional groups. This method is useful to synthesize aldehydes, whereas the Jones and the Sarett-Collins oxidations are better suited to the synthesis of ketones.
PCC is acidic, therefore can react with unstable functional groups. On the other hand, PDC is closer to neutral.
In DMF, the reaction of primary alcohols with PDC (except for ally alcohols) leads to complete oxidation to carboxylic acids.
These chromium-based reagents are used less frequently today with the development of milder and less toxic oxidation reagents.
-
General References
・ PCC: Corey, E. J.; Suggs, J. W. Tetrahedron Lett. 1975, 16, 2647. doi:10.1016/S0040-4039(00)75204-X
・ PDC: Corey, E. J.; Schmidt, G. Tetrahedron Lett. 1979, 20, 399. doi:10.1016/S0040-4039(01)93515-4
・ Ley, S. V.; Madin, A. Comprehensive Organic Synthesis 1991, 7, 253. (Review)
・ Luzzio, F. A. Org. React. 1998, 53, 1. (Review)
-
Reaction Mechanism
The mechanism is similar to that of the Sarett-Collins oxidation.
-
Examples
The synthesis of scopadulcic acid A.[1]
A modification in which a reoxidant such as TMSOOTMS (bistrimethylsilylperoxide) is used to reduce the amount of the chromium is known.
-
Experimental Procedure
-
Experimental Tips
The reaction with PCC or PDC alone generates a tar-like residue as it proceeds, which tends to reduce the yield by sticking to the product. This situation can be improved by adding celite, silica gel, or molecular sieves to the reaction.
-
References
-
Related Books
[amazonjs asin=”3527306420″ locale=”US” title=”Modern Oxidation Methods”]
[amazonjs asin=”B00IGYNTAU” locale=”US” title=”Oxidation and Reduction in Organic Synthesis (Oxford Chemistry Primers) by Donohoe, Timothy J. (2000) Paperback”]

![PCC_PDC_3[1]](https://assets.en.chem-station.com/uploads/2014/04/PCC_PDC_31.gif)
![ol-al-1[1]](https://assets.en.chem-station.com/uploads/2014/04/ol-al-11.gif)