- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The Parikh-Doering oxidation uses SO3•Py (a solid reagent that can be handled easily) as the activator of DMSO. This reaction is especially effective for the oxidation of allylic and secondary alcohols. Unlike the Swern reaction, the reaction works at room temperature and is less prone to the methylthiomethyl ether byproduct formation.
-
General References
・ Parikh, J.P.; Doering, W.E.J. Am. Chem. Soc. 1967, 89, 5505. DOI: 10.1021/ja00997a067
・ Review: Tidwell, T. T. Synthesis 1990, 857. doi:10.1055/s-1990-27036
-
Reaction Mechanism
The basic mechanism is similar to that of the Swern reaction.
-
Examples
Example 1.[1]
Example 2.[2]
Example 3.[3]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Hatakeyama, S.; Sakurai, K.; Numata, H.; Ochi, N.; Takano, S. J. Am. Chem. Soc. 1988, 110, 5201. DOI: 10.1021/ja00223a055
[2] Hauser, F. M.; Hawawasam, P.; Mal, D. J. Am. Chem. Soc. 1988, 110, 2919. DOI: 10.1021/ja00217a038
[3] Hashimoto, S.; Sakata, S.; Sonegawa, M.; Ikegami, S. J. Am. Chem. Soc. 1988, 110, 3670. DOI: 10.1021/ja00219a058
-
Related Books

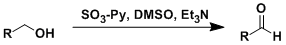
![parlikh_doering_oxi_2[1]](https://assets.en.chem-station.com/uploads/2014/04/parlikh_doering_oxi_21.gif)
![parlikh_doering_oxi_3[1]](https://assets.en.chem-station.com/uploads/2014/04/parlikh_doering_oxi_31.gif)
![parlikh_doering_oxi_4[1]](https://assets.en.chem-station.com/uploads/2014/04/parlikh_doering_oxi_41.gif)
![parlikh_doering_oxi_5[1]](https://assets.en.chem-station.com/uploads/2014/04/parlikh_doering_oxi_51.gif)