- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
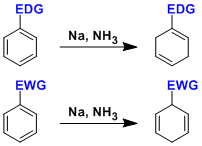
-Aromatic compounds are reduced to 1,4-cyclohexadienes by treatment with alkali matals (Li, Na, or K) or alkaline earth metals (Ca or Mg) dissolved in a mixed solvent of liquid ammonia and alcohol.
-α,β-Unsaturated carbonyl compounds, conjugated dienes, and alkynes are also reduced. The Birch reduction of alkynes give E-olefins selectively. The Birch conditions are also used for the deprotection of benzyl and arylsulfonyl groups.
-Conditions such as Li/DBB (4,4’-di-t-butylbiphenyl) and Na/naphthalene are known as milder alternatives. These conditions are more functional group tolerant than the original Birch conditions.
-Low molecular weight alkyl amines can be used instead of liquid ammonia, in which case the amine serves as a proton source. The reactions can be run at higher temperatures and provide a more strongly reducing system (the Benkeser reduction).
-
General References
・Birch, A. J. J. Chem. Soc. 1944, 430.
・Birch, A. J. J. Chem. Soc. 1945, 809.
・Birch, A. J. J. Chem. Soc. 1946, 593.
・Birch, A. J. J. Chem. Soc. 1947, 102, 1642.
・Birch, A. J. J. Chem. Soc. 1949, 2531.
<Benkeser reduction>
・ Benkeser, R. A.; Robinson, R. E.; Landesman, H. J. Am. Chem. Soc. 1952, 74, 5699. DOI: 10.1021/ja01142a041
・ Benkeser, R. A.; Robinson, R. E.; Sauve, D. M.; Thomas, O. H. J. Am. Chem. Soc. 1955, 77, 3230. DOI: 10.1021/ja01617a025
・ Benkeser, R. A.; Belmonte, F. G.; Kang, J. J. Org. Chem. 1983, 48, 2796. DOI: 10.1021/jo00165a003
<review>
・Watt, G. W. Chem. Rev. 1950, 46, 317. DOI: 10.1021/cr60144a003
・Birch, A.J. Quart. Rev. 1950, 4, 69.
・Birch, A.J.; Smith, H. Quart. Rev. 1958, 12, 17.
・Kaiser, E. M. Synthesis 1972, 391. DOI: 10.1055/s-1972-21889
・Caine, D. Org. React. 1976, 23, 1.
・Hook, J. M.; Mander, L. N. Nat. Prod. Rep. 1986, 3, 35. DOI: 10.1039/NP9860300035
・Schultz, A. G. Acc. Chem. Res. 1990, 23, 207. DOI: 10.1021/ar00175a001
・Mander, L. N. Comprehensive Organic Synthesis 1991, 8, 489.
・Rabideau, P. W.; Marcinow, Z. Org. React. 1992, 42, 1.
・Birch, A. J. Pure Appl. Chem. 1996, 68, 553. doi:10.1351/pac199668030553
・Schultz, A. G. Chem. Commun. 1999, 1263. DOI: 10.1039/A901759C
・Subba Rao, G. S. R. Pure Appl. Chem. 2003, 75, 1443. [PDF]
・Donohoe, T. J.; Thomas, R. E. Nat. Protoc.2007, 2, 1888. doi:10.1038/nprot.2007.245
・Zimmerman, H. E. Acc. Chem. Res. 2012, 45, 164. DOI: 10.1021/ar2000698
-
Reaction Mechanism
The regioselectivity of the Birch reduction is determined by the stability of the anionic intermediates. When the substituent is electron-withdrawing, the anion at the ipso position is stabilized, while the opposite is true when the substituent is electron-donating. (ref: J. Am. Chem. Soc. 1993, 115, 2205., Acc. Chem. Res. 2012, 45, 164.)
The addition of alcohols as a cosolvent helps in preventing undesired isomerizations caused by the strongly basic NH2– anion.
The relative rate for the reduction of benzene is Li(360) > Na(2) > K(1).
-
Examples
The carbanion stabilized by the electron withdrawing groups can be trapped with electrophiles to form carbon-carbon bonds. An example is shown.[1]
-
Experimental Procedure
The Birch reduction of pyrroles.[2]
-
Experimental Tips
-Ammonia boils at about -33˚C. Use a Dewar condenser instead of a regular condenser.
-Teflon-coated stirring bars get corroded and turn black under the Birch conditions. It is better to use glass-coated stirrers.
-Lithium wire is rinsed with pentane to remove the oil first, and then cut by scissors to small pieces.
-The “active” reaction mixture should be colored deep blue.
-Isoprene is sometimes used to quench these one-electron reduction reactions.
-
References
[1] Schultz, A. G.; Pettus, L. J. Org. Chem. 1997, 62, 6855. DOI:10.1021/jo9707592
[2] Donohoe, T. J.; Thomas, R. E. Nat. Protoc. 2007, 2, 1888. doi:10.1038/nprot.2007.245
-
Related Books

![birch_2[1]](https://assets.en.chem-station.com/uploads/2014/04/birch_21.gif)
![birch_3[1]](https://assets.en.chem-station.com/uploads/2014/04/birch_31.gif)
![birch_4[1]](https://assets.en.chem-station.com/uploads/2014/04/birch_41.gif)