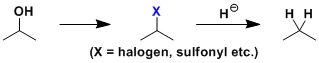- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
Direct reduction of alcohols to alkanes is generally difficult. The conversion usually requires a two-step sequence involving the conversion of alcohols into leaving groups (such as halides and sulfonate esters) followed by reduction with metal hydrides (such as LiAlH4, LiHBEt3, Bu3SnH + radical initiator). There are also other classical methods for reductive removal of halides such as heterogeneous hydrogenation and the Birch reduction.
The reagents such as PX3, PX5, SOCl2, and (COCl)2 provide easy ways for the halogenation step. The Appel conditions are suited when one needs to do the halogenation under neutral conditions. For the sulfonation, MsCl-Et3N and TsCl-Py(-DMAP) are the typical conditions used.
The reduction of alcohols except for aliphatic primary alcohols is known using LiAlH4-AlCl3 (1:3), but the carbocationic intermediates are potentially prone to undesired rearrangements and isomerizations.
The reduction of primary alcohols using NaBH3CN-(PhO)3PCH3I is sometimes used, in which the alkyl iodides formed in situ are reduced in the same pot.
In the popular Barton-McCombie deoxygenation, hydroxyl groups are removed under free-radical based conditions.
-
General References
-
Reaction Mechanism
The mechanism can be regarded as nucleophilic displacement by the hydride (for the reactions using metal hydrides).
![]()
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Books