- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The reaction of aldehydes or ketones with ammonia equivalents or primary or secondary amines in the presence of hydride reducing agents gives alkylated amines.
The reductive amination using sodium cyanoborohydride (NaBH3CN) as the reducing agent is especially called the Borch reaction and has high generality. NaBH3CN is a relatively weak reductant which works under mildly acidic conditions. The control of the pH is important for effecting clean reductive amination. The pH range needed for the reduction of ketones and aldehydes is usually 3~4, whereas that for iminium cations is about 6~7. NaBH3CN works well within this pH range without causing unnecessary side reactions.
NaBH(OAc)3 and 2-picolineborane are used for the same purpose. Both have advantages in that the former has low toxicity and the latter can be used in water.
-
General References
・Borch, R. F.; Bernstein, M. D.; Durst, H. D. J. Am. Chem. Soc. 1971, 93, 2897. DOI: 10.1021/ja00741a013
・Borch, R. F.; Hassid, A. I. J. Org. Chem. 1972, 37, 1673. DOI: 10.1021/jo00975a049
・Emerson, W. S. Org. React. 1990, 14, 174.
・NaBH(OAc)3 conditions: Abdel-Magid, A. F.; Carson, K. G.; Harris, B. D.; Maryanoff, C. A.; Shah, E. D. J. Org. Chem. 1996, 61, 3849. doi: 10.1021/jo960057x
・2-picoline-borane conditions: Sato, S. et al. Tetrahedron 2004, 60, 7899. doi:10.1016/j.tet.2004.06.045
-
Reaction Mechanism
The iminium cation formed by the condensation of a carbonyl compound and an amine reacts with a nucleophilic hydride to give an alkylated amine.
-
Examples
An example of reductive dimethylation of aniline.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Borch, R. F. et al. J. Org. Chem. 1972, 37, 1673. DOI: 10.1021/jo00975a049
-
Related Books

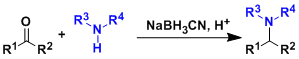
![one-n-1[1]](https://assets.en.chem-station.com/uploads/2014/04/one-n-11.gif)
![one-n-2[1]](https://assets.en.chem-station.com/uploads/2014/04/one-n-21.gif)