- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
Esters, amides, and nitriles are reduced to aldehydes (without overreduction) using appropriate metal hydride reagents at low temperatures. DIBAL and Red-Al are used commonly to serve this purpose.
Stopping the reduction of esters at the aldehyde oxidation state is generally more difficult than that of nitriles. It is usually more reliable to reduce them completely to alcohols and oxidize them back to aldehydes, even though it takes an extra step. An exception is the partial reduction of 5- and 6-membered lactones to the corresponding lactols, which is much easier.
The Weinreb amides and morpholine amides are converted to aldehydes in high yields.
-
General References
-
Reaction Mechanism
-
Examples
The combined use of DIBAL and NaOtBu (“SDBBA”) is known to effect partial reduction of esters at ice-bath temperature.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Song, J. I.; An, D. K. Chem. Lett. 2007, 36, 886. doi:10.1246/cl.2007.886
-
Related Books

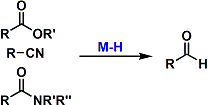
![red_ester_aldehyde_1[1]](https://assets.en.chem-station.com/uploads/2014/04/red_ester_aldehyde_11.gif)