- Generality
- Reagent Availability
- Experimental User Friendliness
- Historical Significance
- Criteria #5
-
General Characteristics
Acid- or base-promoted classical aldol reactions give a complex mixture of products because of the reversibility and the difficulty of controlling the enolate geometry.
In the 1970’s, Mukaiyama’s group developed the crossed aldol reactions using the preformed silyl enol ethers and ketene silyl acetals (both of which are isolable and can be stored for a long time) as nucleophiles.
The Mukaiyama reaction is extremely useful on a lab scale and has been used in numerous complex molecule syntheses.
The reaction usually requires only a catalytic amount of Lewis acid activator. Lewis bases such as fluoride anion can also work as the activator of silyl enol ethers.
Various asymmetric catalysts have been developed and used in many catalytic syntheses of optically active compounds.
-
General References
・ Mukaiyama, T.; Narasaka, K.; Banno, K. Chem. Lett. 1973, 1011. doi:10.1246/cl.1973.1011
・ Mukaiyama, T.; Izawa, T.; Saigo, K. Chem. Lett. 1974, 323. doi:10.1246/cl.1974.323
・ Mukaiyama, T.; Banno, K.; Narasaka, K. J. Am. Chem. Soc. 1974, 96, 7503. DOI: 10.1021/ja00831a019
・ Mukaiyama, T. Org. React. 1982, 28, 203.
・ Mukaiyama, T.; Kobayashi, S. Org. React. 1994, 46, 1. doi: 10.1002/0471264180.or046.01
・ Denmark, S. E.; Stavenger, R. A.; Wong, K.-T. Tetrahedron 1998, 54, 10389. doi:10.1016/S0040-4020(98)00493-1
・ Groger, H.; Vogl, E. M.; Shibasaki, M. Chem. Eur. J. 1998, 4, 1137. [abstract]
・ Mukaiyama, T. Tetrahedron 1999, 55, 8609. doi:10.1016/S0040-4020(99)00437-8
・ Mukaiyama, T. Angew. Chem. Int. Ed. 2004, 43, 5590. doi:10.1002/anie.200300641
-
Reaction Mechanism
Silicon has low Lewis acidity and thus low affinity to carbonyls. The reaction proceeds through a linear transition state because a six-membered transition state cannot be formed. The formation of the syn products is generally favored. However, controlling the stereoselectivity is more difficult than the reactions of lithium and boron enolates because of the higher conformational freedom of the open transition state. Steric and dipole factors greatly influence the stereochemical outcome.
-
Examples
An example of catalytic Mukaiyama aldol reaction.[1]
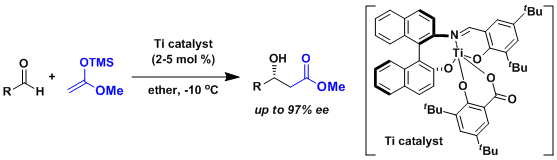
Achieving high stereoselectivity using the enolates derived from acetate is generally considered difficult due to the small steric influence. Carreira developed a catalyst that overcame the problem.[2]
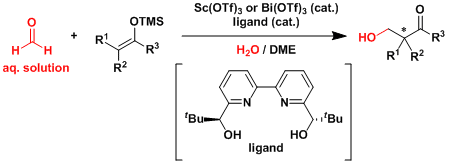
Certain Lewis acidic metal triflates are reasonably stable to water and allows the aqueous solution of formaldehyde to be used in the Mukaiyama aldol reaction.[3]
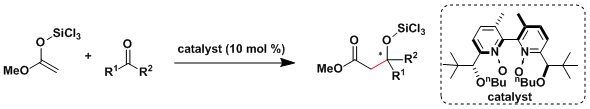
Denmark[4a,b] and Shibasaki[4c] reported catalytic asymmetric aldol additions to unactivated ketones, which are generally less reactive and selective.

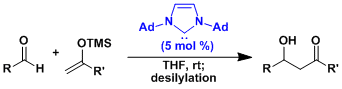
It is extremely challenging to use silyl enol ethers derived from aldehydes in aldol addition. The enol ethers containing the bulky tris(trimethylsilyl)silyl (TTMSS) group prevents the addition to the product, enabling the controlled aldol addition of aldehydic nucleophiles.[5]

An example of Lewis base-activated Mukaiyama aldol reaction.[6]
-
Experimental Procedure
The Mukaiyama aldol reaction using trifluoromethanesulfonimide (HNTf2) catalyst.[7]
-
Experimental Tips
-
References
[1] (a) Mukaiyama, T.; Kobayashi, S.; Uchiro, H.; Shiina, I. Chem. Lett. 1990, 129. doi:10.1246/cl.1990.129 (b) Kobayashi, S.; Fujishita, Y.; Mukaiyama, T. Chem. Lett. 1990, 1455. doi:10.1246/cl.1990.1455
[2] Carreira, E. M. et al. J. Am. Chem. Soc. 1994, 116, 8837. DOI: 10.1021/ja00098a065
[3] (a) Kobayashi, S. et al. J. Am. Chem. Soc. 2004, 126, 12236. DOI: 10.1021/ja047896i (b) Kobayashi, S. et al. Org. Lett. 2005, 7, 4729. DOI: 10.1021/ol051965w
[4] (a) Denmark, S. E.; Fan, Y. J. Am. Chem. Soc. 2002, 124, 4233.[abstract] (b) Denmark, S. E.; Fan, Y., Eastgate, M. D. J. Org. Chem. 2005, 70, 5235. DOI: 10.1021/jo0506276 (c) Oisaki, K.; Zhao, D.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2006, 128, 7164.DOI: 10.1021/ja061815w
[5] (a) Boxer, B. M.; Yamamoto, H. J. Am. Chem. Soc. 2006, 129, 49.DOI: 10.1021/ja054725k (b) idem. J. Am. Chem. Soc. 2007,129, 2762. DOI: 10.1021/ja0693542 (c) Boxer, M. B.; Akakura, M.; Yamamoto, H. J. Am. Chem. Soc. 2008,130, 1580. DOI: 10.1021/ja7102586 (d) Boxer, M. B.; Yamamoto, H. Org. Lett. 2008, 10, 453. DOI: 10.1021/ol702825p
[6] Song, J. J.; Tan, Z.; Reeves, J. T.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2007, 9, 1013. DOI: 10.1021/ol0630494
[7] Saito, S.; Nakadai, M.; Yamamoto, H. Synlett 2001, 1245. DOI: 10.1055/s-2001-16055
-
Related Books
[amazonjs asin=”3527307141″ locale=”US” title=”Modern Aldol Reactions (2 Volume Set)”]

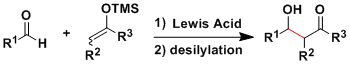
![mukaiyama_aldol_12[1]](https://assets.en.chem-station.com/uploads/2014/04/mukaiyama_aldol_121.gif)
![mukaiyama_aldol_2[1]](https://assets.en.chem-station.com/uploads/2014/04/mukaiyama_aldol_21.gif)
![mukaiyama_aldol_3[1]](https://assets.en.chem-station.com/uploads/2014/04/mukaiyama_aldol_31.gif)
![mukaiyama_aldol_4[1]](https://assets.en.chem-station.com/uploads/2014/04/mukaiyama_aldol_41.gif)
![mukaiyama_aldol_8[1]](https://assets.en.chem-station.com/uploads/2014/04/mukaiyama_aldol_81.gif)