- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
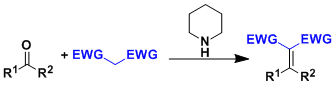
Activated methylene compounds condense with aldehydes and ketones to give substituted alkenes. Piperidine is generally used as the catalyst. Nitromethane also undergoes similar reaction to give nitroolefins.
In the Doebner modification, the decarboxylative condensation of malonic acid and aldehydes mediated by pyridine gives α,β-unsaturated acids.
-
General References
・Knoevenagel, E. Ber. Deutsch. Chem. Ges. 1898, 31, 2596. doi:10.1002/cber.18980310308
・Doebner Modification: Doebner, O. Ber. Deutsch. Chem. Ges. 1900, 33, 2140. doi:10.1002/cber.190203501187
・Johnson, J. R. Org. React. 1942, 1, 210.
・Jones, G. Org. React. 1967, 15, 204.
・Tietze, L. F.; Beifuss, U. Comprehensive Organic Synthesis 1991, 2, 341.
-
Reaction Mechanism
-
Examples
The synthesis of gelsemine.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Fukuyama, T.; Liu, G. J. Am. Chem. Soc. 1996, 118, 7426. DOI: 10.1021/ja961701s
-
Related Books

![knoevenagel_condense_4[1]](https://assets.en.chem-station.com/uploads/2014/04/knoevenagel_condense_41.gif)
![kno02[1]](https://assets.en.chem-station.com/uploads/2014/04/kno021.gif)
![knoevenagel_3[1]](https://assets.en.chem-station.com/uploads/2014/04/knoevenagel_31.gif)