- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
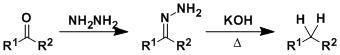
The deoxygenation of carbonyl compounds through hydrazone formation is known as the Wolff-Kishner reduction.
Heating a mixture containing an aldehyde or a ketone, hydrazine, ethylene glycol, and a base (NaOH or KOH) gives a simple hydrocarbon (the Huang-Minglon modification). The reaction conditions are strongly basic, which is complementary to the similar Clemmensen reduction, which is carried out under acidic conditions.
Semicarbazones and azines also work in place of hydrazones to effect the same transformation.
-
General References
・Kishner, N. J. Russ. Phys. Chem. Soc. 1911, 43, 582.
・Wolff, L. Ann. 1912, 394, 86.
・Huang-Minlon Modification: (a) Minlon, H. J. Am. Chem. Soc. 1946, 68, 2487. DOI: 10.1021/ja01216a013 (b) idem. J. Am. Chem. Soc. 1949, 71, 3301. DOI: 10.1021/ja01178a008 (c) Hunig, S.; Lucke, E.; Brenningesr, W. Org. Synth. 1963, 43, 34.
・Todd, D. Org. React. 1948, 4, 378.
・Szmant, H. H. Angew. Chem., Int. Ed. Engl. 1968, 7, 120.
・Hutchins, R. O.; Hutchins, M. K. Comprehensive Organic Synthesis 1991, 8, 327.
-
Reaction Mechanism
The protonations of the carbonyl carbon is considered to be the rate-limiting step.
-
Examples
A typical example showing chemoselectivity.
A modification in which tosylhydrazine and sodium cyanoborohydride are used to convert ketones into alkanes directly is known.[1]
The decomposition of hydrazones can be done at room temperature using potassium t-butoxide in DMSO-tBuOH. The bis-TBS hydrazine reagent for efficient hydrazone formation is also known (the Cram-Myers modification).[2]
An application during the synthesis of neotripterifordin.[3]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Hutchins, R. O.; Milewski, C. A.; Maryanoff, B. E. J. Am. Chem. Soc. 1973, 95, 3662. DOI: 10.1021/ja00792a03
[2] Furrow, M. E.; Myers, A. G. J. Am. Chem. Soc. 2004, 126, 5436. DOI: 10.1021/ja049694s
[3] Corey, E. J.; Liu, K. J. Am. Chem. Soc. 1997, 119, 9929. DOI: 10.1021/ja972549c
-
Related Books

![al-ane2[1]](https://assets.en.chem-station.com/uploads/2014/04/al-ane21.gif)
![al-ane3[1]](https://assets.en.chem-station.com/uploads/2014/04/al-ane31.gif)
![wolff_kishner_4[1]](https://assets.en.chem-station.com/uploads/2014/04/wolff_kishner_41.gif)
![wolff_kishner_6[1]](https://assets.en.chem-station.com/uploads/2014/04/wolff_kishner_61.gif)