- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
Periodic acid or sodium periodate is used to oxidatively cleave 1,2-diols, producing a pair of aldehydes or ketones.
Sodium periodate also works as the reoxidant of osmium tetroxide, so these two reagents provide a way to oxidatively cleave alkenes under mild conditions.
Aqueous solvent systems are generally used for the solubility reason. Silica gel supported NaIO4 is available when one needs to use nonpolar solvents.
-
General References
・Malaprade, L. Bull. Soc. Chim. France 1928, 43, 683.;Compt. Rend. 1928, 186, 382.
-
Reaction Mechanism
-
Examples
Under the conditions consisting of OsO4(cat.)/NaIO4/2,6-lutidine, olefins can be cleaved easily. The addition of lutidine suppresses the formation of α-hydroxyketone byproduct.[1] ![malaprade_2[1]](https://assets.en.chem-station.com/uploads/2014/05/malaprade_21.gif)
The synthesis of terpestacin.[2]
The oxidative cleavage of partially protected mannitol.[3]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Yu, W.; Mei, Y.; Kang, Y.; Hua, Z.; Jin, Z. Org. Lett. 2004, 6, 3217. DOI: 10.1021/ol0400342
[2] Trost, B. M.; Dong, G.; Vance, J. A. J. Am. Chem. Soc. 2007, 129. 4540. DOI: 10.1021/ja070571s
[3] Schmid, C. R. ; Bryant J. D. Org. Synth. 1995, 72, 6. [PDF]
-
Related Books

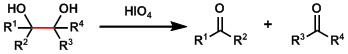
![malaprade_3[1]](https://assets.en.chem-station.com/uploads/2014/05/malaprade_31.gif)
![malaprade_4[1]](https://assets.en.chem-station.com/uploads/2014/05/malaprade_41.gif)
![malaprade_5[1]](https://assets.en.chem-station.com/uploads/2014/05/malaprade_51.gif)