- Generality
- Reagent Availability
- Experimental User Friendliness
- Safety and Environmental Impact
- Criteria #5
-
General Characteristics
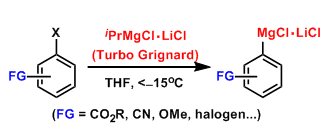
The addition of one equivalent of lithium chloride when preparing Grignard reagents accelerates both the metal-halogen exchange and the insertion of magnesium. RMgCl-LiCl is known as the Turbo Grignard reagent as it allows facile preparation of Grignard reagents at low temperatures.
The preparation of organomagnesium compounds containing normally reactive functional groups such as esters, nitriles, ketones, and various unstable heteroaromatics became possible.
This reagent has become a valuable tool in organic synthesis for its utility at practical temperatures and causing less side reactions during metal-halogen exchange.
-
General References
Krasovskiy, A.; Knochel, P. Angew. Chem. Int. Ed. 2004, 43, 3333. DOI: 10.1002/anie.200454084
-
Reaction Mechanism
The dissociation of the Grignard aggregates and the formation of highly anionic i-PrMgCl-LiCl are considered as a rationale for the acceleration of the reaction.
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Books