Lo, J. C.; Yabe, Y.; Baran, P. S. J. Am. Chem. Soc. 2014, ASAP.
DOI: 10.1021/ja4117632
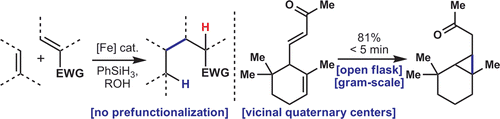
A redox-economic method for the direct coupling of olefins that uses an inexpensive iron catalyst and a silane reducing agent is reported. Thus, unactivated olefins can be joined directly to electron-deficient olefins in both intra- and intermolecular settings to generate hindered bicyclic systems, vicinal quaternary centers, and even cyclopropanes in good yield. The reaction is not sensitive to oxygen or moisture and has been performed on gram-scale. Most importantly, it allows access to many compounds that would be difficult or perhaps impossible to access using other methods.
Two-phase terpene total synthesis has been demonstrated to be a useful strategy to access steroids, diterpenes, and sesquiterpenes.[1] The approach consists of a cyclase phase, where a lowly oxidized terpene skeleton is rapidly constructed from inexpensive starting materials, and an oxidase phase, where a series of site-selective oxidations furnishes the desired natural product. Phil Baran laboratory, The Scripps Research Institute, is interested in pursuing such a strategy toward the ent-kaurane family of diterpenoids . During these synthetic study, they discovered a practical method for the reductive coupling of olefins that utilizes a readily available and inexpensive Fe source as a catalyst.
In the presence of catalytic Fe(acac)3, and PhSiH3 as a reductant in EtOH/(CH2OH)2, unactivated olefins can be connected directly with electron-defficient olefines in box intra- and intermolecular fashion. Details and inside story of this coupling shows in their blog (A Tales of Olefins). BTW, they seems to made chemdraw figures slender from Corey’s style.
- References
[1] “Two-Phase Terpene Total Synthesis: Historical Perspective and Application to the Taxol® Problem”
Ishihara, Y.; Baran, P. Synlett 2010, 2010, 1733–1745. DOI: 10.1055/s-0030-1258123
This account presents our laboratory’s latest endeavors in Csp3−H activation in the context of total synthesis, as well as a historical perspective of the field of Csp3−H activation. Our viewpoints in using a two-phase terpene synthesis strategy and how known oxidative transformations could benefit an eventual biomimetic synthesis of Taxol® are discussed.
- Related Links
Baran Lab
OpenFlask (Baran lab Blog)
- Related Books
[amazonjs asin=”3642768377″ locale=”US” title=”Synthesis of Marine Natural Products 1: Terpenoids (Bioorganic Marine Chemistry)”][amazonjs asin=”3642454097″ locale=”US” title=”Chemistry of Plant Natural Products: Stereochemistry, Conformation, Synthesis, Biology and Medicine”]