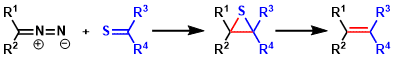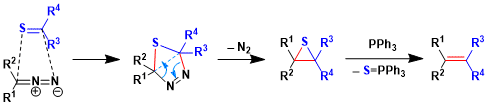- Popularity
- Responsibility
- Atom efficiency
- Criteria #4
- Criteria #5
-
Characteristics
The Barton-Kellogg reaction is a coupling reaction between a ketone and a thioketone through a diazo intermediate forming an alkene. The McMurry Reaction is similar to the Barton-Kellogg reaction. However, the McMurry. Reaction is unsuccessful for using two different ketone. In this regard, this coupling is cross-coupling rather than a homo coupling.
-
Literature reference
・Staudinger, H.; Siegwart, J. Helv Chim. Acta 1920, 3, 833. DOI:10.1002/hlca.19200030178
・Barton, D. H. R.; Willis, B. J. J. Chem. Soc. D 1970, 1225. DOI:10.1039/C29700001225
・Kellogg, R. M.; Wassenaar, S. Tetrahedron Lett. 1970, 11, 1987. DOI:10.1016/S0040-4039(01)98134-1
・Kellogg, R. M. Tetrahedron 1976, 32, 2165. DOI:10.1016/0040-4020(76)85131-9
-
Reaction mechanism
The 1,3-dipolar cycloaddition of diazo compound with the thioketone gives thiadiazoline intermediate. This intermediate is unstable and it forms a stable episulfide by explosion of N2 gas. Triphenylphosphine opens the three-menbered ring and then form alkene and triphenyl phosphinesulfoxide that is similar as Wittig reaction.
-
Example of reactions
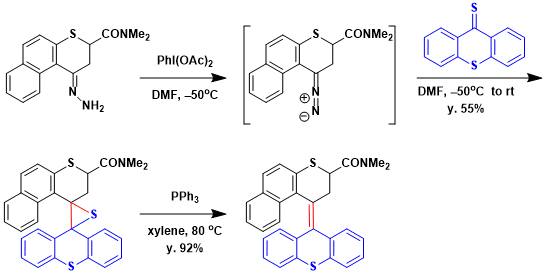
Feringa and coworkers applied Barton–Kellogg to the synthesis of a molecular motor.[1] coupling Treatment of diazoketone with freshly prepared thioketone led to a Barton–Kellogg coupling reaction generating episulfide in 55% yield. Desulfurization of episulfide was achieved in refluxing p-xylene in the presence of PPh3 which generated overcrowded alkene in 92% yield.
-
Procedure
A solution of hydrazone (0.55 g, 1.8 mmol) in DMF (10 mL) was cooled to -50oC and iodobenzene diacetate (0.59 g, 1.8 mmol) in DMF (2 mL) was added. After stirring for approximately 3 min to generate diazo compound, a solution of 9H-thioxanthene-9-thione (0.331 g, 1.45 mmol) in DMF (5 mL) was added and the cooling bath removed. The reaction mixture was left stirring at rt for 5 h. Additional ethyl acetate (50 mL) was added and the mixture was washed with water (5×50 mL) and, brine (150 mL), dried (Na2SO4) and concentrated in vacuo to give the crude product. Column chromatography (silica gel; ethyl acetate: pentane = 1:4 as an eluent, Rf = 0.45) provided episulfide (0.50 g, 1.0 mmol, 55 %) as a light yellow oil.
A solution of episulfide (258 mg, 0.520 mmol) was heated at 80°C in p-xylene (5 mL) in the presence of triphenylphosphine (163 mg, 0.62 mmol) for 12 h. After cooling to rt, the p-xylene was removed under reduced pressure and the remaining oil was purified by column chromatography (silica gel; ethyl acetate: pentane = 1:4, Rf = 0.42) after which a racemic mixture of alkene was obtained as a yellow solid (222 mg, 0.48 mmol, 92 %).
-
Bibliography
[1] “Reversing the direction in a light-driven rotary molecular motor”
Ruangsupapichat, N.; Pollard, M. M.; Harutyunyan, S. R.; Feringa, B. L. Nat. Chem. 2011, 3, 53. doi:10.1038/nchem.872
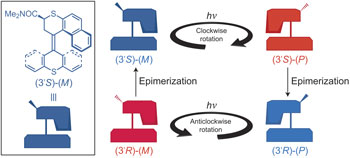
Biological rotary motors can alter their mechanical function by changing the direction of rotary motion. Achieving a similar reversal of direction of rotation in artificial molecular motors presents a fundamental stereochemical challenge: how to change from clockwise to anticlockwise motion without compromising the autonomous unidirectional rotary behaviour of the system. A new molecular motor with multilevel control of rotary motion is reported here, in which the direction of light-powered rotation can be reversed by base-catalysed epimerization. The key steps are deprotonation and reprotonation of the photochemically generated less-stable isomers during the 3608 unidirectional rotary cycle, with complete inversion of the configuration at the stereogenic centre. The ability to change directionality is an essential step towards mechanical molecular systems with adaptive functional behaviour.
- Related Books
-
Related Links
Barton–Kellogg reaction: Wikipedia
Barton Olefin Synthesis (Barton Kellogg Reaction)
Barton Olefin Synthesis (Barton-Kellogg Reaction)