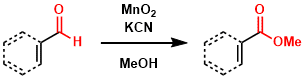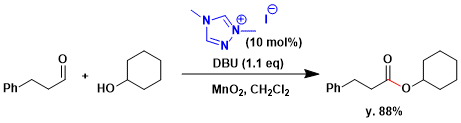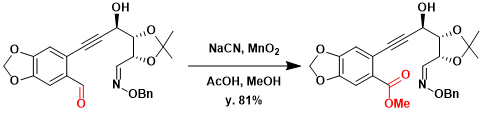- Popularity
- Responsibility
- Versatility
- Gentleness
- Criteria #5
-
Characteristics
The Corey-Gilman-Ganem oxidation transforms benzylic or α,β-unsaturated aldehydes into the corresponding methyl esters. The aldehydes can be selectively oxidized to esters in presence of MnO2 and hydrogen cyanide in methanol at ambient temperature. Cis/trans isomerization, a problem when other reagents such as basic silver oxide are employed, is avoided. However, conjugate addition of cyanide ion can be sometimes problematic. Recently, N-Heterocyclic carbenes instead of MCN can be catalyzed the oxidation of unactivated aldehydes to esters with manganese(IV) oxide.
-
Literature reference
・Corey, E. J.; Gilman, N. W.; Ganem, B. E. J. Am. Chem. Soc. 1968, 90, 5616. DOI:10.1021/ja01022a059
・Gilman, N. W. Chem Commun. 1971, 733. DOI:10.1039/C29710000733
・Foota, J. S.; Kannoa, H.; Giblin, G. M.P.; Taylor, R. J. K. Synthesis 2003, 1055. DOI:10.1055/s-2003-39163
<NHC-catalyzed conditions>
・Chan, A.; Scheidt, K. A. Org. Lett. 2005, 7, 905. DOI: 10.1021/ol050100f
・Maki, B. E.; Chan, A.; Phillips, E. M.; Scheidt, K. A. Org. Lett. 2007, 9, 371. DOI:10.1021/ol062940f
・Maki, B. E.; Scheidt, K. A. Org. Lett. 2008, 10, 4331. DOI:10.1021/ol8018488
・Maki, B. E.; Chan, A.; Phillips, E. A.; Scheidt, K. A. Tetrahedron 2009, 65, 3102. DOI:10.1016/j.tet.2008.10.033
・De Sarkar, S.; Grimme, S.; Studer, A. J. Am. Chem. Soc. 2010, 132, 1190. DOI: 10.1021/ja910540j
・Mohlmann, L.; Ludwig, S.; Blechert, S. Beilstein J. Org. Chem. 2013, 9, 602. DOI:10.3762/bjoc.9.65
-
Reaction mechanism
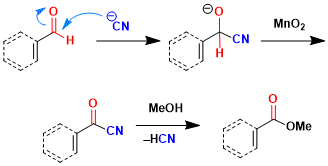
The aldehyde undergoes reaction with HCN to give cyanohydrins, which proceeds further oxidation to acyl cyanide. The latter on alcoholysis leads to corresponding benzylic or α,β-unsaturated carboxylic ester.
-
Example of reactions
N-Heterocyclic carbenes derived from benzimidazolium salts are effective catalysts for Corey-Gilman-Ganem oxidation.[1] In 2008, Scheidt reported the general oxidation of saturated aldehydes to esters using NHC-carbene catalysis.[1c] On the other hand, 1.0 equivalent of NaCN with MnO2 and the aldehyde does not provide any saturated ester.
In the total synthesis of (-)-Lycoricidine, (+)-Lycoricidine, and (+)-Narciclasine by Keck, hydroxyaldehyde was converted (81%isolated yield) to the hydroxy ester using the Corey- Gilman-Ganem oxidation.[2]
-
Procedure[2]
To a stirring solution of acetic acid (0.338 g, 5.62 mmol) in 12 mL of MeOH were added sodium cyanide (0.538 g, 11.1 mmol), a solution of aldehyde (0.971 g, 2.22 mmol in 5 mL of MeOH) as a 2.7:1 mixture of oxide isomers via cannula (wash, 2 3 mL), and precipitated activated manganese dioxide (3.85 g, 44.4 mmol). The reaction mixture was allowed to stir at rt for 16 h, at which time it was concentrated to near dryness under reduced pressure, then diluted with 100 mL of Et2O and washed with 50 mL of water. The layers were separated, and the aqueous layer was back-extracted with Et2O (3 100 mL). The combined organic layers were dried over Na2SO4, filtered through a pad of Celite (1 6 cm), and concentrated under reduced pressure. Purification was accomplished by flash column chromatography using a 3.21cm column, eluting with a gradient of 200 mL each of 20%, 30%, 40%, and 50% EtOAc/hexanes, collecting 25 mL fractions. The product-containing fractions (22–35) were collected and concentrated to give the ester 18 (0.836 g, 81% yield) as a 1.5:1 mixture of oxide isomers and as a light yellow foam.
-
Bibliography
[1] (a) Chan, A.; Scheidt, K. A. Org. Lett. 2005, 7, 905. DOI: 10.1021/ol050100f (b) Maki, B. E.; Chan, A.; Phillips, E. M.; Scheidt, K. A. Org. Lett. 2007, 9, 371. DOI:10.1021/ol062940f (c) Maki, B. E.; Scheidt, K. A. Org. Lett. 2008, 10, 4331. DOI:10.1021/ol8018488 (d) Maki, B. E.; Chan, A.; Phillips, E. A.; Scheidt, K. A. Tetrahedron 2009, 65, 3102. DOI:10.1016/j.tet.2008.10.033
[2] Keck, G. E.; Wager, T. T.; Rodriquez, J. F. D. J. Am. Chem. Soc. 1999, 121, 5176. DOI: 10.1021/ja9826688
- Related Books
[amazonjs asin=”3527323201″ locale=”US” title=”Modern Oxidation Methods”][amazonjs asin=”1119953278″ locale=”US” title=”Handbook of Reagents for Organic Synthesis: Catalytic Oxidation Reagents (Hdbk of Reagents for Organic Synthesis)”]
-
Related Links
Corey-Gilman-Ganem Oxidataion - Wikipedia Oxidation (Myers' Group, PDF)