Dielmann, F.; Andrada, D. M.; Frenking, G.; Bertrand, G. J. Am. Chem. Soc., 2014. 136, 3800.
DOI: 10.1021/ja5007355
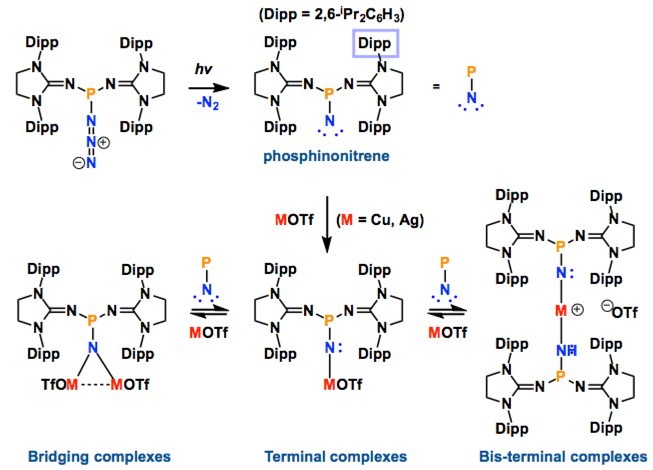
Transition metal complexes featuring a metal−nitrogen multiple bond have been widely studied due to their implication in dinitrogen fixation and catalytic nitrogen−carbon bond formation. Terminal copper− and silver−nitrene complexes have long been proposed to be the key intermediates in aziridination and amination reactions using azides as the nitrogen source. However, due to their high reactivity, these species have eluded isolation and spectroscopic characterization even at low temperatures. In this paper we report that a stable phosphinonitrene reacts with coinage metal trifluoromethanesulfonates, affording bridging and terminal copper− and silver−nitrene complexes, which are characterized by NMR spectroscopy.
Recent successful isolation of a singlet phosphinonitrene by Bertrand group has opened a door for major advance in nitrene chemistry [1,2], which reminds us the beginning of carbene chemistry around in 1988~1991.
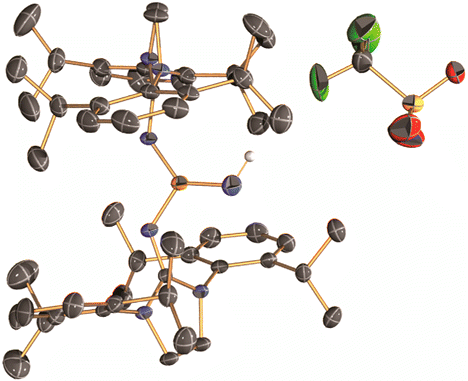
In this report, they introduced the nitrene as a ligand to form coinage metal complexes. Terminal nitrene-copper and -silver species have been believed to be the significant intermediates in mostly nitrene transfer reactions. By X-ray diffraction analysis, they unambiguously reveal the molecular structures of the bridging and terminal Cu & Ag−nitrene complexes. Importantly, the results demonstrate that nitrene can donate not only two electrons but also four electrons to metal depending on the coordination modes, despite the labile property of M-N bond. Further reactivity of these complexes will be explored as Bertrand emphasizes.
-
References
[1] “A Crystalline Singlet Phosphinonitrene: A Nitrogen Atom–Transfer Agent”
Dielmann, F.; Back, O.; Henry-Ellinger, M.; Jerabek, P.; Frenking, G.; Bertrand, G. Science, 2012. 337, 1526 – 1528. DOI: 10.1126/science.1226022
A variety of transition metal–nitrido complexes (metallonitrenes) have been isolated and studied in the context of modeling intermediates in biological nitrogen fixation by the nitrogenase enzymes and the industrial Haber-Bosch hydrogenation of nitrogen gas into ammonia. In contrast, nonmetallic nitrenes have so far only been spectroscopically observed at low temperatures, despite their intermediacy in a range of organic reactions. Here, we report the synthesis of a bis(imidazolidin-2-iminato)phosphinonitrene, which is stable at room temperature in solution and can even be isolated in the solid state. The bonding between phosphorus and nitrogen is analogous to that observed for metallonitrenes. We also show that this nitrido phosphorus derivative can be used to transfer a nitrogen atom to organic fragments, a difficult task for transition metal–nitrido complexes.
[2] “Crystalline, Lewis Base-Free, Cationic Phosphoranimines (Iminophosphonium Salts)”
Dielmann, F.; Moore, C. E.; Rheingold, A. L.; Frenking, G.; Bertrand, G. J. Am. Chem. Soc., 2013. 135, 14071 – 14073. DOI: 10.1021/ja4080979
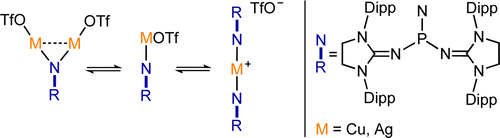
 Cationic phosphoranimines have been postulated as intermediates in phosphazene polymerization chemistry. However, the high electrophilicity of the phosphorus center has so far prevented their characterization. Here, we report the synthesis of two Lewis basefree iminophosphonium salts, obtained by reaction of a stable phosphinonitrene with methyl trifluoromethanesulfonate and trifluoromethanesulfonic acid. These cationic species were characterized by NMR spectroscopy and single-crystal X-ray diffraction analysis.
Cationic phosphoranimines have been postulated as intermediates in phosphazene polymerization chemistry. However, the high electrophilicity of the phosphorus center has so far prevented their characterization. Here, we report the synthesis of two Lewis basefree iminophosphonium salts, obtained by reaction of a stable phosphinonitrene with methyl trifluoromethanesulfonate and trifluoromethanesulfonic acid. These cationic species were characterized by NMR spectroscopy and single-crystal X-ray diffraction analysis.
-
Related Books
[amazonjs asin=”0824708318″ locale=”US” title=”Carbene Chemistry: From Fleeting Intermediates to Powerful Reagents”]
-
Related Links
Bertrand Lab home Page