Kuprat, M.; Schulz, A.; Villinger, A. Angew. Chem. Int. Ed. 52, 2013. 7126 – 7130.
DOI: 10.1002/anie.201302725
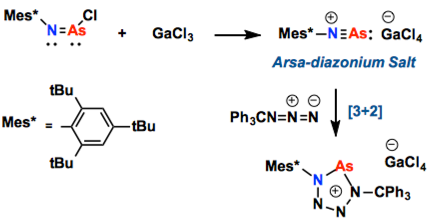
Arsa-diazonium salts containing a cation with an arsenic–nitrogen triple bond ([R-N≡As]+), which is stable at room temperature, are isolated and fully characterized for the first time. Analogous to the diazonium salts, arsa-diazonium salts can be used in [3+2] dipolar cycloaddition reactions with azides to prepare arsenic analogues (RAsN4) of pentazoles (RN5).
Schulz and Villinger groups have widely developed a chemistry of fundamental compounds containing group 15 elements; especially they are recognized as specialists handling heavier pnictogen such as phosphorus, arsenic, antimony, and bismuth.
Since the collapse of “double bond rule” in 1981 by West group’s isolation of Si=Si species [1], a variety of compounds featuring unsaturated bonds with heavy atoms have been isolated up to date. In this report, the isolation of new member of the triple bond species involving N≡As is described. Remarkably, Schulz group demonstrated that it can be used like diazonium salts. Due to the high reactivity, it act as an efficient dipolarophile in [3+2] cycloadditions to produce five-membered ring derivatives just as found in click chemistry, but here the reaction proceeded under low temperature without any catalysts.
-
References
[1] “Tetramesityldisilene, a Stable Compound Containing a Silicon-Silicon Double Bond”
West, R.; Fink, M. J.; Michl, J. Science. 1981, 214, 1343 – 1344.DOI: 10.1126/science.214.4527.1343
Irradiation of 2,2-bis(2,4,6-trimethylphenyl)hexamethyltrisilane in hydrocarbon solution produces tetramesityldisilene, which can be isolated as a yellow-orange solid stable to room temperature and above in the absence of air. Like the olefins of carbon chemistry, tetramesityldisilene undergoes addition reactions across the silicon-silicon double bond.
-
Related Links
Schulz Lab home Page